Smad3 Sensitizes Hepatocelluar Carcinoma Cells to Cisplatin by Repressing Phosphorylation of AKT
- PMID: 27110775
- PMCID: PMC4849060
- DOI: 10.3390/ijms17040610
Smad3 Sensitizes Hepatocelluar Carcinoma Cells to Cisplatin by Repressing Phosphorylation of AKT
Abstract
Background: Heptocelluar carcinoma (HCC) is insensitive to chemotherapy due to limited bioavailability and acquired drug resistance. Smad3 plays dual roles by inhibiting cell growth initially and promoting the progression of advanced tumors in HCC. However, the role of smad3 in chemosensitivity of HCC remains elusive.
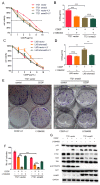
Methods: The role of smad3 in chemosensitivity of HCC was measured by cell viability, apoptosis, plate colony formation assays and xenograft tumor models. Non-smad signaling was detected by Western blotting to search for the underlying mechanisms.
Results: Smad3 enhanced the chemosensitivity of HCC cells to cisplatin. Smad3 upregulated p21(Waf1/Cip1) and downregulated c-myc and bcl2 with the treatment of cisplatin. Moreover, overexpression of smad3 repressed the phosphorylation of AKT, and vice versa. Inhibition of PI3K/AKT pathway by LY294002 restored chemosensitivity of smad3-deficiency cells to cisplatin in HCC.
Conclusion: Smad3 sensitizes HCC cells to the effects of cisplatin by repressing phosphorylation of AKT and combination of inhibitor of AKT pathway and conventional chemotherapy may be a potential way to solve drug resistance in HCC.
Keywords: AKT; LY294002; cisplatin; drug resistance; hepatocelluar carcinoma; smad3.
Figures



Similar articles
-
Mucin1 shifts Smad3 signaling from the tumor-suppressive pSmad3C/p21(WAF1) pathway to the oncogenic pSmad3L/c-Myc pathway by activating JNK in human hepatocellular carcinoma cells.Oncotarget. 2015 Feb 28;6(6):4253-65. doi: 10.18632/oncotarget.2973. Oncotarget. 2015. PMID: 25714018 Free PMC article.
-
Erinacine Facilitates the Opening of the Mitochondrial Permeability Transition Pore Through the Inhibition of the PI3K/ Akt/GSK-3β Signaling Pathway in Human Hepatocellular Carcinoma.Cell Physiol Biochem. 2018;50(3):851-867. doi: 10.1159/000494472. Epub 2018 Oct 24. Cell Physiol Biochem. 2018. Retraction in: Cell Physiol Biochem. 2021;55(5):674. doi: 10.33594/000000464. PMID: 30355923 Retracted.
-
LY‑294002 enhances the chemosensitivity of liver cancer to oxaliplatin by blocking the PI3K/AKT/HIF‑1α pathway.Mol Med Rep. 2021 Jul;24(1):508. doi: 10.3892/mmr.2021.12147. Epub 2021 May 13. Mol Med Rep. 2021. PMID: 33982772 Free PMC article.
-
Linker phosphorylation of Smad3 promotes fibro-carcinogenesis in chronic viral hepatitis of hepatocellular carcinoma.World J Gastroenterol. 2014 Nov 7;20(41):15018-27. doi: 10.3748/wjg.v20.i41.15018. World J Gastroenterol. 2014. PMID: 25386050 Free PMC article. Review.
-
Novel Investigations of Flavonoids as Chemopreventive Agents for Hepatocellular Carcinoma.Biomed Res Int. 2015;2015:840542. doi: 10.1155/2015/840542. Epub 2015 Dec 16. Biomed Res Int. 2015. PMID: 26858957 Free PMC article. Review.
Cited by
-
microRNA-17 functions as an oncogene by downregulating Smad3 expression in hepatocellular carcinoma.Cell Death Dis. 2019 Sep 26;10(10):723. doi: 10.1038/s41419-019-1960-z. Cell Death Dis. 2019. PMID: 31558704 Free PMC article.
-
Downregulation of TGF-β1 suppressed proliferation and increased chemosensitivity of ovarian cancer cells by promoting BRCA1/Smad3 signaling.Biol Res. 2018 Dec 29;51(1):58. doi: 10.1186/s40659-018-0205-4. Biol Res. 2018. PMID: 30594239 Free PMC article.
-
Smad3 deficiency promotes beta cell proliferation and function in db/db mice via restoring Pax6 expression.Theranostics. 2021 Jan 1;11(6):2845-2859. doi: 10.7150/thno.51857. eCollection 2021. Theranostics. 2021. PMID: 33456576 Free PMC article.
-
MEIS-1 level in unresectable hepatocellular carcinoma can predict the post-treatment outcomes of radiofrequency ablation.Oncotarget. 2018 Jan 11;9(20):15252-15265. doi: 10.18632/oncotarget.24165. eCollection 2018 Mar 16. Oncotarget. 2018. PMID: 29632641 Free PMC article.
-
AF1q Mediates Tumor Progression in Colorectal Cancer by Regulating AKT Signaling.Int J Mol Sci. 2017 May 5;18(5):987. doi: 10.3390/ijms18050987. Int J Mol Sci. 2017. PMID: 28475127 Free PMC article.
References
-
- Poon R.T., Fan S.T., Lo C.M., Liu C.L., Wong J. Long-term survival and pattern of recurrence after resection of small hepatocellular carcinoma in patients with preserved liver function: Implications for a strategy of salvage transplantation. Ann. Surg. 2002;235:373–382. doi: 10.1097/00000658-200203000-00009. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

