Endothelial Notch1 Is Required for Proper Development of the Semilunar Valves and Cardiac Outflow Tract
- PMID: 27107132
- PMCID: PMC4843530
- DOI: 10.1161/JAHA.115.003075
Endothelial Notch1 Is Required for Proper Development of the Semilunar Valves and Cardiac Outflow Tract
Abstract
Background: Congenital heart disease is the most common type of birth defect, affecting ≈2% of the population. Malformations involving the cardiac outflow tract and semilunar valves account for >50% of these cases predominantly because of a bicuspid aortic valve, which has an estimated prevalence of 1% in the population. We previously reported that mutations in NOTCH1 were a cause of bicuspid aortic valve in nonsyndromic autosomal-dominant human pedigrees. Subsequently, we described a highly penetrant mouse model of aortic valve disease, consisting of a bicuspid aortic valve with thickened cusps and associated stenosis and regurgitation, in Notch1-haploinsufficient adult mice backcrossed into a Nos3-null background.
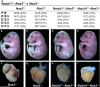
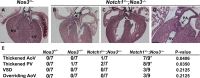
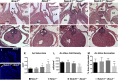
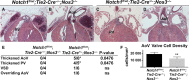
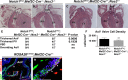
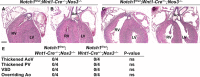
Methods and results: Here, we described the congenital cardiac abnormalities in Notch1(+/-);Nos3(-/-) embryos that led to ≈65% lethality by postnatal day 10. Although expected Mendelian ratios of Notch1(+/-);Nos3(-/-) embryos were found at embryonic day 18.5, histological examination revealed thickened, malformed semilunar valve leaflets accompanied by additional anomalies of the cardiac outflow tract including ventricular septal defects and overriding aorta. The aortic valve leaflets of Notch1(+/-);Nos3(-/-) embryos at embryonic day 15.5 were significantly thicker than controls, consistent with a defect in remodeling of the semilunar valve cushions. In addition, we generated mice haploinsufficient for Notch1 specifically in endothelial and endothelial-derived cells in a Nos3-null background and found that Notch1(fl/+);Tie2-Cre(+/-);Nos3(-/-) mice recapitulate the congenital cardiac phenotype of Notch1(+/-);Nos3(-/-) embryos.
Conclusions: Our data demonstrate the role of endothelial Notch1 in the proper development of the semilunar valves and cardiac outflow tract.
Keywords: Notch1 signaling; bicuspid aortic valve; cardiovascular genetics; congenital heart defect; conotruncal heart defects.
© 2016 The Authors. Published on behalf of the American Heart Association, Inc., by Wiley Blackwell.
Figures
Similar articles
-
Bicuspid aortic valve formation: Nos3 mutation leads to abnormal lineage patterning of neural crest cells and the second heart field.Dis Model Mech. 2018 Oct 19;11(10):dmm034637. doi: 10.1242/dmm.034637. Dis Model Mech. 2018. PMID: 30242109 Free PMC article.
-
Deficient signaling via Alk2 (Acvr1) leads to bicuspid aortic valve development.PLoS One. 2012;7(4):e35539. doi: 10.1371/journal.pone.0035539. Epub 2012 Apr 19. PLoS One. 2012. PMID: 22536403 Free PMC article.
-
Disrupted Slit-Robo signalling results in membranous ventricular septum defects and bicuspid aortic valves.Cardiovasc Res. 2015 Apr 1;106(1):55-66. doi: 10.1093/cvr/cvv040. Epub 2015 Feb 17. Cardiovasc Res. 2015. PMID: 25691540 Free PMC article.
-
Notch Signaling in Aortic Valve Development and Disease.2016 Jun 25. In: Nakanishi T, Markwald RR, Baldwin HS, Keller BB, Srivastava D, Yamagishi H, editors. Etiology and Morphogenesis of Congenital Heart Disease: From Gene Function and Cellular Interaction to Morphology [Internet]. Tokyo: Springer; 2016. Chapter 53. 2016 Jun 25. In: Nakanishi T, Markwald RR, Baldwin HS, Keller BB, Srivastava D, Yamagishi H, editors. Etiology and Morphogenesis of Congenital Heart Disease: From Gene Function and Cellular Interaction to Morphology [Internet]. Tokyo: Springer; 2016. Chapter 53. PMID: 29787141 Free Books & Documents. Review.
-
Embryonic development of bicuspid aortic valves.Prog Cardiovasc Dis. 2020 Jul-Aug;63(4):407-418. doi: 10.1016/j.pcad.2020.06.008. Epub 2020 Jun 25. Prog Cardiovasc Dis. 2020. PMID: 32592706 Review.
Cited by
-
Growth and maturation of heart valves leads to changes in endothelial cell distribution, impaired function, decreased metabolism and reduced cell proliferation.J Mol Cell Cardiol. 2016 Nov;100:72-82. doi: 10.1016/j.yjmcc.2016.10.006. Epub 2016 Oct 15. J Mol Cell Cardiol. 2016. PMID: 27756541 Free PMC article.
-
Evaluating High-Confidence Genes in Conotruncal Cardiac Defects by Gene Burden Analyses.J Am Heart Assoc. 2023 Feb 21;12(4):e028226. doi: 10.1161/JAHA.122.028226. Epub 2023 Feb 15. J Am Heart Assoc. 2023. PMID: 36789878 Free PMC article.
-
Genetic basis of aortic valvular disease.Curr Opin Cardiol. 2017 May;32(3):239-245. doi: 10.1097/HCO.0000000000000384. Curr Opin Cardiol. 2017. PMID: 28157139 Free PMC article.
-
Decoding Genetics of Congenital Heart Disease Using Patient-Derived Induced Pluripotent Stem Cells (iPSCs).Front Cell Dev Biol. 2021 Jan 21;9:630069. doi: 10.3389/fcell.2021.630069. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33585486 Free PMC article. Review.
-
Multi-Omics Approaches to Define Calcific Aortic Valve Disease Pathogenesis.Circ Res. 2021 Apr 30;128(9):1371-1397. doi: 10.1161/CIRCRESAHA.120.317979. Epub 2021 Apr 29. Circ Res. 2021. PMID: 33914608 Free PMC article. Review.
References
-
- Verma S, Siu SC. Aortic dilatation in patients with bicuspid aortic valve. N Engl J Med. 2014;370:1920–1929. - PubMed
-
- Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez‐Sarano M. Burden of valvular heart diseases: a population‐based study. Lancet. 2006;368:1005–1011. - PubMed
-
- Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39:1890–1900. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

