SHREC Silences Heterochromatin via Distinct Remodeling and Deacetylation Modules
- PMID: 27105116
- PMCID: PMC4890606
- DOI: 10.1016/j.molcel.2016.03.016
SHREC Silences Heterochromatin via Distinct Remodeling and Deacetylation Modules
Abstract
Nucleosome remodeling and deacetylation (NuRD) complexes are co-transcriptional regulators implicated in differentiation, development, and diseases. Methyl-CpG binding domain (MBD) proteins play an essential role in recruitment of NuRD complexes to their target sites in chromatin. The related SHREC complex in fission yeast drives transcriptional gene silencing in heterochromatin through cooperation with HP1 proteins. How remodeler and histone deacetylase (HDAC) cooperate within NuRD complexes remains unresolved. We determined that in SHREC the two modules occupy distant sites on the scaffold protein Clr1 and that repressive activity of SHREC can be modulated by the expression level of the HDAC-associated Clr1 domain alone. Moreover, the crystal structure of Clr2 reveals an MBD-like domain mediating recruitment of the HDAC module to heterochromatin. Thus, SHREC bi-functionality is organized in two separate modules with separate recruitment mechanisms, which work together to elicit transcriptional silencing at heterochromatic loci.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures
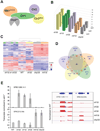



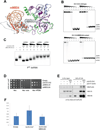


Similar articles
-
The Mi-2 homolog Mit1 actively positions nucleosomes within heterochromatin to suppress transcription.Mol Cell Biol. 2014 Jun;34(11):2046-61. doi: 10.1128/MCB.01609-13. Epub 2014 Mar 24. Mol Cell Biol. 2014. PMID: 24662054 Free PMC article.
-
SHREC, an effector complex for heterochromatic transcriptional silencing.Cell. 2007 Feb 9;128(3):491-504. doi: 10.1016/j.cell.2006.12.035. Cell. 2007. PMID: 17289569
-
Transcriptional gene silencing requires dedicated interaction between HP1 protein Chp2 and chromatin remodeler Mit1.Genes Dev. 2019 May 1;33(9-10):565-577. doi: 10.1101/gad.320440.118. Epub 2019 Feb 26. Genes Dev. 2019. PMID: 30808655 Free PMC article.
-
Studies on the mechanism of RNAi-dependent heterochromatin assembly.Cold Spring Harb Symp Quant Biol. 2006;71:461-71. doi: 10.1101/sqb.2006.71.044. Cold Spring Harb Symp Quant Biol. 2006. PMID: 17381328 Review.
-
[RNAi and the formation of heterochromatin in Schizosaccharomyces pombe.].J Soc Biol. 2007;201(4):401-10. doi: 10.1051/jbio:2007901. Epub 2008 Mar 5. J Soc Biol. 2007. PMID: 18533101 Review. French.
Cited by
-
Tracking live-cell single-molecule dynamics enables measurements of heterochromatinassociated protein-protein interactions.bioRxiv [Preprint]. 2023 Oct 19:2023.03.08.531771. doi: 10.1101/2023.03.08.531771. bioRxiv. 2023. Update in: Nucleic Acids Res. 2024 Oct 14;52(18):10731-10746. doi: 10.1093/nar/gkae692 PMID: 36945633 Free PMC article. Updated. Preprint.
-
The euchromatic histone mark H3K36me3 preserves heterochromatin through sequestration of an acetyltransferase complex in fission yeast.Microb Cell. 2020 Jan 3;7(3):80-92. doi: 10.15698/mic2020.03.711. Microb Cell. 2020. PMID: 32161768 Free PMC article.
-
CHD Chromatin Remodeling Protein Diversification Yields Novel Clades and Domains Absent in Classic Model Organisms.Genome Biol Evol. 2022 May 3;14(5):evac066. doi: 10.1093/gbe/evac066. Genome Biol Evol. 2022. PMID: 35524943 Free PMC article.
-
Breakers and amplifiers in chromatin circuitry: acetylation and ubiquitination control the heterochromatin machinery.Curr Opin Struct Biol. 2021 Dec;71:156-163. doi: 10.1016/j.sbi.2021.06.012. Epub 2021 Jul 22. Curr Opin Struct Biol. 2021. PMID: 34303934 Free PMC article. Review.
-
Native Chromatin Proteomics Reveals a Role for Specific Nucleoporins in Heterochromatin Organization and Maintenance.Mol Cell. 2020 Jan 2;77(1):51-66.e8. doi: 10.1016/j.molcel.2019.10.018. Epub 2019 Nov 26. Mol Cell. 2020. PMID: 31784357 Free PMC article.
References
-
- Allshire RC, Nimmo ER, Ekwall K, Javerzat JP, Cranston G. Mutations derepressing silent centromeric domains in fission yeast disrupt chromosome segregation. Genes Dev. 1995;9:218–233. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

