Tissue expression profile of human neonatal Fc receptor (FcRn) in Tg32 transgenic mice
- PMID: 27104806
- PMCID: PMC4968098
- DOI: 10.1080/19420862.2016.1178436
Tissue expression profile of human neonatal Fc receptor (FcRn) in Tg32 transgenic mice
Abstract
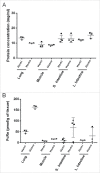
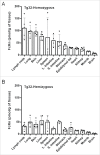
The neonatal Fc receptor (FcRn) is a homeostatic receptor responsible for prolonging immunoglobulin G (IgG) half-life by protecting it from lysosomal degradation and recycling it to systemic circulation. Tissue-specific FcRn expression is a critical parameter in physiologically-based pharmacokinetic (PBPK) modeling for translational pharmacokinetics of Fc-containing biotherapeutics. Using online peptide immuno-affinity chromatography coupled with high resolution mass spectrometry, we established a quantitative FcRn tissue protein expression profile in human FcRn (hFcRn) transgenic mice, Tg32 homozygous and hemizygous strains. The concentration of hFcRn across 14 tissues ranged from 3.5 to 111.2 pmole per gram of tissue. Our hFcRn quantification data from Tg32 mice will enable a more refined PBPK model to improve the accuracy of human PK predictions for Fc-containing biotherapeutics.
Keywords: Antibody; IA-LC-HRMS; PBPK models; Tg32; human FcRn; tissue-based target quantification; transgenic mice.
Figures
Similar articles
-
Quantitative Analysis of Human Neonatal Fc Receptor (FcRn) Tissue Expression in Transgenic Mice by Online Peptide Immuno-Affinity LC-HRMS.Anal Chem. 2016 Apr 19;88(8):4239-47. doi: 10.1021/acs.analchem.5b03900. Epub 2016 Apr 4. Anal Chem. 2016. PMID: 27012525
-
Distribution of FcRn Across Species and Tissues.J Histochem Cytochem. 2017 Jun;65(6):321-333. doi: 10.1369/0022155417705095. Epub 2017 Apr 12. J Histochem Cytochem. 2017. PMID: 28402755 Free PMC article.
-
Utility of a human FcRn transgenic mouse model in drug discovery for early assessment and prediction of human pharmacokinetics of monoclonal antibodies.MAbs. 2016 Aug-Sep;8(6):1064-78. doi: 10.1080/19420862.2016.1193660. Epub 2016 May 27. MAbs. 2016. PMID: 27232760 Free PMC article.
-
Are endosomal trafficking parameters better targets for improving mAb pharmacokinetics than FcRn binding affinity?Mol Immunol. 2013 Dec;56(4):660-74. doi: 10.1016/j.molimm.2013.05.008. Epub 2013 Aug 2. Mol Immunol. 2013. PMID: 23917469 Review.
-
Neonatal Fc receptor (FcRn): a novel target for therapeutic antibodies and antibody engineering.J Drug Target. 2014 May;22(4):269-78. doi: 10.3109/1061186X.2013.875030. Epub 2014 Jan 9. J Drug Target. 2014. PMID: 24404896 Review.
Cited by
-
Physiologically-based modeling of monoclonal antibody pharmacokinetics in drug discovery and development.Drug Metab Pharmacokinet. 2019 Feb;34(1):3-13. doi: 10.1016/j.dmpk.2018.11.002. Epub 2018 Nov 22. Drug Metab Pharmacokinet. 2019. PMID: 30522890 Free PMC article. Review.
-
Cross-species/cross-modality physiologically based pharmacokinetics for biologics: 89Zr-labelled albumin-binding domain antibody GSK3128349 in humans.MAbs. 2020 Jan-Dec;12(1):1832861. doi: 10.1080/19420862.2020.1832861. MAbs. 2020. PMID: 33073698 Free PMC article.
-
Human FcRn Tissue Expression Profile and Half-Life in PBMCs.Biomolecules. 2019 Aug 15;9(8):373. doi: 10.3390/biom9080373. Biomolecules. 2019. PMID: 31443181 Free PMC article.
-
Quantification of IgG monoclonal antibody clearance in tissues.MAbs. 2017 Aug/Sep;9(6):1007-1015. doi: 10.1080/19420862.2017.1337619. Epub 2017 Jun 14. MAbs. 2017. PMID: 28613103 Free PMC article.
-
Mechanistic incorporation of FcRn binding in plasma and endosomes in a whole body PBPK model for large molecules.J Pharmacokinet Pharmacodyn. 2023 Jun;50(3):229-241. doi: 10.1007/s10928-023-09849-9. Epub 2023 Mar 6. J Pharmacokinet Pharmacodyn. 2023. PMID: 36877385
References
-
- Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol 2007; 7:715-25; PMID:17703228; http://dx.doi.org/10.1038/nri2155 - DOI - PubMed
-
- Junghans RP, Anderson CL. The protection receptor for IgG catabolism is the beta2-microglobulin-containing neonatal intestinal transport receptor. Proc Natl Acad Sci U S A 1996; 93:5512-6; PMID:8643606; http://dx.doi.org/10.1073/pnas.93.11.5512 - DOI - PMC - PubMed
-
- Challa DK, Velmurugan R, Ober RJ, Sally Ward E. FcRn: from molecular interactions to regulation of IgG pharmacokinetics and functions. Curr Top Microbiol Immunol 2014; 382:249-72; PMID:25116104; http://dx.doi.org/10.1007/978-3-319-07911-0_12 - PubMed
-
- Jones HM, Chen Y, Gibson C, Heimbach T, Parrott N, Peters SA, Snoeys J, Upreti VV, Zheng M, Hall SD. Physiologically based pharmacokinetic modeling in drug discovery and development: a pharmaceutical industry perspective. Clin Pharmacol Ther 2015; 97:247-62; PMID:25670209; http://dx.doi.org/10.1002/cpt.37 - DOI - PubMed
-
- Ferl GZ, Wu AM, DiStefano JJ 3rd. A predictive model of therapeutic monoclonal antibody dynamics and regulation by the neonatal Fc receptor (FcRn). Ann Biomed Eng 2005; 33:1640-52; PMID:16341929; http://dx.doi.org/10.1007/s10439-005-7410-3 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
