The transcription factor RUNX2 regulates receptor tyrosine kinase expression in melanoma
- PMID: 27102439
- PMCID: PMC5045426
- DOI: 10.18632/oncotarget.8822
The transcription factor RUNX2 regulates receptor tyrosine kinase expression in melanoma
Abstract
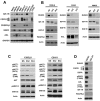
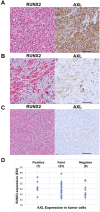
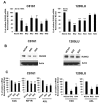
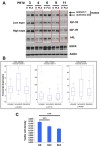
Receptor tyrosine kinases-based autocrine loops largely contribute to activate the MAPK and PI3K/AKT pathways in melanoma. However, the molecular mechanisms involved in generating these autocrine loops are still largely unknown. In the present study, we examine the role of the transcription factor RUNX2 in the regulation of receptor tyrosine kinase (RTK) expression in melanoma. We have demonstrated that RUNX2-deficient melanoma cells display a significant decrease in three receptor tyrosine kinases, EGFR, IGF-1R and PDGFRβ. In addition, we found co-expression of RUNX2 and another RTK, AXL, in both melanoma cells and melanoma patient samples. We observed a decrease in phosphoAKT2 (S474) and phosphoAKT (T308) levels when RUNX2 knock down resulted in significant RTK down regulation. Finally, we showed a dramatic up regulation of RUNX2 expression with concomitant up-regulation of EGFR, IGF-1R and AXL in melanoma cells resistant to the BRAF V600E inhibitor PLX4720. Taken together, our results strongly suggest that RUNX2 might be a key player in RTK-based autocrine loops and a mediator of resistance to BRAF V600E inhibitors involving RTK up regulation in melanoma.
Keywords: RUNX2; melanoma; receptor tyrosine kinase; resistance to targeted therapy; transcription factor.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures


Similar articles
-
RUNX2 is overexpressed in melanoma cells and mediates their migration and invasion.Cancer Lett. 2014 Jun 28;348(1-2):61-70. doi: 10.1016/j.canlet.2014.03.011. Epub 2014 Mar 18. Cancer Lett. 2014. PMID: 24657655 Free PMC article.
-
The transcription cofactor c-JUN mediates phenotype switching and BRAF inhibitor resistance in melanoma.Sci Signal. 2015 Aug 18;8(390):ra82. doi: 10.1126/scisignal.aab1111. Sci Signal. 2015. PMID: 26286024
-
A melanoma subtype with intrinsic resistance to BRAF inhibition identified by receptor tyrosine kinases gene-driven classification.Oncotarget. 2015 Mar 10;6(7):5118-33. doi: 10.18632/oncotarget.3007. Oncotarget. 2015. PMID: 25742786 Free PMC article.
-
RUNX2 and the PI3K/AKT axis reciprocal activation as a driving force for tumor progression.Mol Cancer. 2015 Jul 25;14:137. doi: 10.1186/s12943-015-0404-3. Mol Cancer. 2015. PMID: 26204939 Free PMC article. Review.
-
Receptor tyrosine kinases and their activation in melanoma.Pigment Cell Melanoma Res. 2011 Jun;24(3):446-61. doi: 10.1111/j.1755-148X.2011.00836.x. Epub 2011 Mar 3. Pigment Cell Melanoma Res. 2011. PMID: 21320293 Review.
Cited by
-
RUNX2/CBFB modulates the response to MEK inhibitors through activation of receptor tyrosine kinases in KRAS-mutant colorectal cancer.Transl Oncol. 2020 Feb;13(2):201-211. doi: 10.1016/j.tranon.2019.10.006. Epub 2019 Dec 20. Transl Oncol. 2020. PMID: 31865182 Free PMC article.
-
Inhibition of human cervical cancer cell invasion by IL-37 involving runt related transcription factor 2 suppression.Ann Transl Med. 2019 Oct;7(20):568. doi: 10.21037/atm.2019.09.38. Ann Transl Med. 2019. PMID: 31807549 Free PMC article.
-
RUNX1/EGFR pathway contributes to STAT3 activation and tumor growth caused by hyperactivated mTORC1.Mol Ther Oncolytics. 2021 Oct 28;23:387-401. doi: 10.1016/j.omto.2021.10.009. eCollection 2021 Dec 17. Mol Ther Oncolytics. 2021. PMID: 34853810 Free PMC article.
-
NRF2 Enables EGFR Signaling in Melanoma Cells.Int J Mol Sci. 2021 Apr 7;22(8):3803. doi: 10.3390/ijms22083803. Int J Mol Sci. 2021. PMID: 33916908 Free PMC article.
-
Mechanisms of Acquired BRAF Inhibitor Resistance in Melanoma: A Systematic Review.Cancers (Basel). 2020 Sep 29;12(10):2801. doi: 10.3390/cancers12102801. Cancers (Basel). 2020. PMID: 33003483 Free PMC article. Review.
References
-
- Blyth K, Vaillant F, Jenkins A, McDonald L, Pringle MA, Huser C, Stein T, Neil J, Cameron ER. Runx2 in normal tissues and cancer cells: A developing story. Blood Cells Mol Dis. 2010;45:117–123. - PubMed
-
- Karsenty G, Kronenberg HM, Settembre C. Genetic control of bone formation. Annual review of cell and developmental biology. 2009;25:629–648. - PubMed
-
- Provot S, Schipani E. Molecular mechanisms of endochondral bone development. Biochemical and biophysical research communications. 2005;328:658–665. - PubMed
-
- Mackie EJ, Tatarczuch L, Mirams M. The skeleton: a multi-functional complex organ: the growth plate chondrocyte and endochondral ossification. The Journal of endocrinology. 2011;211:109–121. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

