Cost-Effectiveness of Cetuximab for Advanced Esophageal Squamous Cell Carcinoma
- PMID: 27100871
- PMCID: PMC4839693
- DOI: 10.1371/journal.pone.0153943
Cost-Effectiveness of Cetuximab for Advanced Esophageal Squamous Cell Carcinoma
Abstract
Background: Costly biologicals in palliative oncology are emerging at a rapid pace. For example, in patients with advanced esophageal squamous cell carcinoma addition of cetuximab to a palliative chemotherapy regimen appears to improve survival. However, it simultaneously results in higher costs. We aimed to determine the incremental cost-effectiveness ratio of adding cetuximab to first-line chemotherapeutic treatment of patients with advanced esophageal squamous cell carcinoma, based on data from a randomized controlled phase II trial.
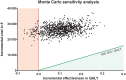
Methods: A cost effectiveness analysis model was applied based on individual patient data. It included only direct medical costs from the health-care perspective. Quality-adjusted life-years and incremental cost-effectiveness ratios were calculated. Sensitivity analysis was performed by a Monte Carlo analysis.
Results: Adding cetuximab to a cisplatin-5-fluorouracil first-line regimen for advanced esophageal squamous cell carcinoma resulted in an the incremental cost-effectiveness ratio of €252,203 per quality-adjusted life-year. Sensitivity analysis shows that there is a chance of less than 0.001 that the incremental cost-effectiveness ratio will be less than a maximum willingness to pay threshold of €40,000 per quality-adjusted life-year, which is representative for the threshold used in The Netherlands and other developed countries.
Conclusions: Addition of cetuximab to a cisplatin-5-fluorouracil first-line regimen for advanced esophageal squamous cell carcinoma is not cost-effective when appraised according to currently accepted criteria. Cost-effectiveness analyses using outcome data from early clinical trials (i.c. a phase II trial) enable pharmaceutical companies and policy makers to gain early insight into whether a new drug meets the current eligibility standards for reimbursement and thereby potential admittance for use in regular clinical practice.
Conflict of interest statement
Figures

Similar articles
-
Real-world cost-effectiveness of cetuximab in locally advanced squamous cell carcinoma of the head and neck.Eur Arch Otorhinolaryngol. 2015 Aug;272(8):2007-16. doi: 10.1007/s00405-014-3106-3. Epub 2014 Jun 19. Eur Arch Otorhinolaryngol. 2015. PMID: 24943191
-
Cost effectiveness of cetuximab concurrent with radiotherapy for patients with locally advanced head and neck cancer in Taiwan: a decision-tree analysis.Clin Drug Investig. 2011 Oct 1;31(10):717-26. doi: 10.2165/11588980-000000000-00000. Clin Drug Investig. 2011. PMID: 21744880
-
Cost-Effectiveness Analysis of Toripalimab Plus Paclitaxel and Cisplatin as First-Line Treatment for Advanced or Metastatic Esophageal Squamous Cell Carcinoma.Adv Ther. 2023 Mar;40(3):1019-1030. doi: 10.1007/s12325-022-02402-z. Epub 2023 Jan 9. Adv Ther. 2023. PMID: 36622553
-
Cetuximab for the treatment of recurrent and/or metastatic squamous cell carcinoma of the head and neck.Health Technol Assess. 2009 Oct;13 Suppl 3:49-54. doi: 10.3310/hta13suppl3/08. Health Technol Assess. 2009. PMID: 19846029 Review.
-
The clinical effectiveness and cost-effectiveness of cetuximab (review of technology appraisal no. 176) and panitumumab (partial review of technology appraisal no. 240) for previously untreated metastatic colorectal cancer: a systematic review and economic evaluation.Health Technol Assess. 2017 Jun;21(38):1-294. doi: 10.3310/hta21380. Health Technol Assess. 2017. PMID: 28682222 Free PMC article. Review.
Cited by
-
Cost-Effectiveness Analysis of Camrelizumab Immunotherapy versus Docetaxel or Irinotecan Chemotherapy as Second-Line Therapy for Advanced or Metastatic Esophageal Squamous Cell Carcinoma.Cancer Manag Res. 2021 Nov 2;13:8219-8230. doi: 10.2147/CMAR.S335515. eCollection 2021. Cancer Manag Res. 2021. PMID: 34754242 Free PMC article.
-
Cost-Effectiveness of Nivolumab Immunotherapy vs. Paclitaxel or Docetaxel Chemotherapy as Second-Line Therapy in Advanced Esophageal Squamous Cell Carcinoma in China.Front Public Health. 2022 Jun 29;10:923619. doi: 10.3389/fpubh.2022.923619. eCollection 2022. Front Public Health. 2022. PMID: 35844891 Free PMC article.
-
How are we evaluating the cost-effectiveness of companion biomarkers for targeted cancer therapies? A systematic review.BMC Cancer. 2021 Sep 1;21(1):980. doi: 10.1186/s12885-021-08725-4. BMC Cancer. 2021. PMID: 34470603 Free PMC article.
-
Cost-effectiveness analysis of serplulimab in combination with cisplatin plus 5-fluorouracil chemotherapy compared to cisplatin plus 5-fluorouracil chemotherapy as first-line treatment for advanced or metastatic esophageal squamous cell carcinoma in China.Ther Adv Med Oncol. 2023 Nov 22;15:17588359231213621. doi: 10.1177/17588359231213621. eCollection 2023. Ther Adv Med Oncol. 2023. PMID: 38028139 Free PMC article.
-
Incorporating Pharmacometrics into Pharmacoeconomic Models: Applications from Drug Development.Pharmacoeconomics. 2020 Oct;38(10):1031-1042. doi: 10.1007/s40273-020-00944-0. Pharmacoeconomics. 2020. PMID: 32734572 Free PMC article.
References
-
- Rickwood S, Kleinrock M, Núñez-Gaviria M, IMS Institute for Healthcare Informatics. The Global Use of Medicines: Outlook through 2017. Available: http://www.imshealth.com/2013.
-
- Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349(23):2241–52. - PubMed
-
- Polednak AP. Trends in survival for both histologic types of esophageal cancer in US surveillance, epidemiology and end results areas. Int J Cancer. 2003;105(1):98–100. - PubMed
-
- Lorenzen S, Schuster T, Porschen R, Al-Batran SE, Hofheinz R, Thuss-Patience P, et al. Cetuximab plus cisplatin-5-fluorouracil versus cisplatin-5-fluorouracil alone in first-line metastatic squamous cell carcinoma of the esophagus: a randomized phase II study of the Arbeitsgemeinschaft Internistische Onkologie. Ann Oncol. 2009;20(10):1667–73. 10.1093/annonc/mdp069 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

