Is It Possible to Shorten the Duration of Adjuvant Chemotherapy for Locally Advanced Rectal Cancer?
- PMID: 27100436
- PMCID: PMC4845840
- DOI: 10.1097/MD.0000000000003427
Is It Possible to Shorten the Duration of Adjuvant Chemotherapy for Locally Advanced Rectal Cancer?
Abstract
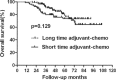
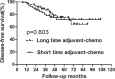
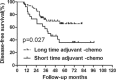
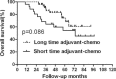
The long duration of 4 months of postoperative adjuvant chemotherapy is currently recommended for locally advanced rectal cancer after preoperative chemoradiation and surgery. Whether a short duration could be applied in these patients is unknown. So, the purpose of this study is to evaluate the effects on prognosis based on different durations of adjuvant chemotherapy for rectal cancer. We performed a retrospective study of 200 rectal cancer patients who were treated with preoperative chemoradiation and were pathologically graded as ypII and ypIII stages between March 2003 and May 2012. All patients were divided into 2 groups according to the median duration of adjuvant chemotherapy of 2 months. Overall survival (OS) and disease-free survival (DFS) were compared between patients with duration shorter and longer than 2 months in the whole group and subgroups of ypII and ypIII. Recurrence patterns were also analyzed in all subgroups. Multivariate analysis was performed to explore clinical factors that were significantly associated with DFS, local recurrence-free survival, and distant metastasis-free survival. In subgroup of ypII stage, the 5-year OS and DFS were similar between patients in long and short durations of adjuvant chemotherapy. For patients of ypIII stage, although no significant difference was found in OS between patients in short and long durations, DFS was showed to be higher in the group of long duration. Further analysis showed that longer duration of adjuvant chemotherapy could lead to improved control of distant metastasis and no impact on local control. Multivariable analysis indicated that long duration of adjuvant chemotherapy is significantly associated with longer distant metastasis-free survival in patients with ypIII stage, but not in those with ypII stage. A long duration of at least 2 months of postoperative adjuvant chemotherapy is necessary for patients with ypIII stage, whereas it may not be absolutely appropriate for those with ypII stage. Therefore, we suggest a tailored selection of durations of adjuvant chemotherapy for locally advanced rectal cancer.
Conflict of interest statement
Conflict of interest statement: The authors have declared no conflicts of interest.
Figures


Similar articles
-
Tailored selection of the interval between neoadjuvant chemoradiotherapy and surgery for locally advanced rectal cancer: analysis based on the pathologic stage or chemoradiation response.J Cancer Res Clin Oncol. 2015 Apr;141(4):719-28. doi: 10.1007/s00432-014-1843-8. Epub 2014 Oct 9. J Cancer Res Clin Oncol. 2015. PMID: 25296560
-
Does downstaging predict improved outcome after preoperative chemoradiation for extraperitoneal locally advanced rectal cancer? A long-term analysis of 165 patients.Int J Radiat Oncol Biol Phys. 2002 Jul 1;53(3):664-74. doi: 10.1016/s0360-3016(02)02764-5. Int J Radiat Oncol Biol Phys. 2002. PMID: 12062610
-
Prognostic implications of response to preoperative infusional chemoradiation in locally advanced rectal cancer.Radiother Oncol. 1999 May;51(2):153-60. doi: 10.1016/s0167-8140(99)00054-7. Radiother Oncol. 1999. PMID: 10435807
-
Locally advanced rectal cancer: a comparison of management strategies.Drugs. 2011 Jun 18;71(9):1153-77. doi: 10.2165/11591330-000000000-00000. Drugs. 2011. PMID: 21711061 Review.
-
Postoperative adjuvant chemotherapy for stage II colorectal cancer: a systematic review of 12 randomized controlled trials.J Gastrointest Surg. 2012 Mar;16(3):646-55. doi: 10.1007/s11605-011-1682-8. Epub 2011 Dec 23. J Gastrointest Surg. 2012. PMID: 22194062 Review.
Cited by
-
Factors associated with metachronous metastases and survival in locally advanced and recurrent rectal cancer.BJS Open. 2020 Aug 28;4(6):1172-9. doi: 10.1002/bjs5.50341. Online ahead of print. BJS Open. 2020. PMID: 32856767 Free PMC article.
-
Long-term outcomes of surgery alone versus surgery following preoperative chemoradiotherapy for early T3 rectal cancer: A propensity score analysis.Medicine (Baltimore). 2017 Mar;96(12):e6362. doi: 10.1097/MD.0000000000006362. Medicine (Baltimore). 2017. PMID: 28328820 Free PMC article.
-
Oxaliplatin-containing adjuvant chemotherapy improves the survival of locally advanced rectal cancer patients with pathological complete response after pre-operative chemoradiotherapy.Gastroenterol Rep (Oxf). 2018 Aug;6(3):195-201. doi: 10.1093/gastro/goy009. Epub 2018 Apr 27. Gastroenterol Rep (Oxf). 2018. PMID: 30151204 Free PMC article.
References
-
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: rectal cancer, 2015. http://www.nccn.org/. - PubMed
-
- Bujko K, Glynne-Jones R, Bujko M. Does adjuvant fluoropyrimidine-based chemotherapy provide a benefit for patients with resected rectal cancer who have already received neoadjuvant radiochemotherapy? A systematic review of randomised trials. Ann Oncol 2010; 21:1743–1750. - PubMed
-
- Bosset JF, Collette L, Calais G, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med 2006; 355:1114–1123. - PubMed
-
- Govindarajan A, Reidy D, Weiser MR, et al. Recurrence rates and prognostic factors in ypN0 rectal cancer after neoadjuvant chemoradiation and total mesorectal excision. Ann Surg Oncol 2011; 18:3666–3672. - PubMed
-
- Huh JW, Kim HR. Postoperative chemotherapy after neo-adjuvant chemoradiation and surgery for rectal cancer: Is it essential for patients with ypT0–2N0? J Surg Oncol 2009; 100:387–391. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

