Expression Patterns of Organic Anion Transporting Polypeptides 1B1 and 1B3 Protein in Human Pediatric Liver
- PMID: 27098745
- PMCID: PMC4931892
- DOI: 10.1124/dmd.115.069252
Expression Patterns of Organic Anion Transporting Polypeptides 1B1 and 1B3 Protein in Human Pediatric Liver
Abstract
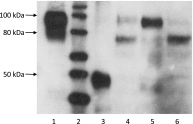
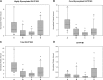
Determining appropriate pharmacotherapy in young children can be challenging due to uncertainties in the development of drug disposition pathways. With knowledge of the ontogeny of drug-metabolizing enzymes and an emerging focus on drug transporters, the developmental pattern of the uptake transporters organic anion transporting polypeptide (OATP) 1B1 and 1B3 was assessed by relative protein quantification using Western blotting in 80 human pediatric liver specimens covering an age range from 9 days to 12 years. OATP1B3 exhibited high expression at birth, which declined over the first months of life, and then increased again in the preadolescent period. In comparison with children 6-12 years of age, the relative protein expression of highly glycosylated (total) OATP1B3 was 235% (357%) in children <3 months of age, 33% (64%) in the age group from 3 months to 2 years, and 50% (59%) in children 2-6 years of age. The fraction of highly glycosylated to total OATP1B3 increased with age, indicating ontogenic processes not only at the transcriptional level but also at the post-translational level. Similar to OATP1B3, OATP1B1 showed high interindividual variability in relative protein expression but no statistically significant difference among the studied age groups.
Copyright © 2016 by The American Society for Pharmacology and Experimental Therapeutics.
Figures
Similar articles
-
Treatment with proteasome inhibitor bortezomib decreases organic anion transporting polypeptide (OATP) 1B3-mediated transport in a substrate-dependent manner.PLoS One. 2017 Nov 6;12(11):e0186924. doi: 10.1371/journal.pone.0186924. eCollection 2017. PLoS One. 2017. PMID: 29107984 Free PMC article.
-
Interaction of Sulfonylureas with Liver Uptake Transporters OATP1B1 and OATP1B3.Basic Clin Pharmacol Toxicol. 2018 Aug;123(2):147-154. doi: 10.1111/bcpt.12992. Epub 2018 Apr 10. Basic Clin Pharmacol Toxicol. 2018. PMID: 29498478
-
Human organic anion transporting polypeptide 1B3 (OATP1B3) is more heavily N-glycosylated than OATP1B1 in extracellular loops 2 and 5.Int J Biol Macromol. 2024 Oct;278(Pt 2):134748. doi: 10.1016/j.ijbiomac.2024.134748. Epub 2024 Aug 13. Int J Biol Macromol. 2024. PMID: 39147348
-
Genetic polymorphisms of uptake (OATP1B1, 1B3) and efflux (MRP2, BCRP) transporters: implications for inter-individual differences in the pharmacokinetics and pharmacodynamics of statins and other clinically relevant drugs.Expert Opin Drug Metab Toxicol. 2009 Jul;5(7):703-29. doi: 10.1517/17425250902976854. Expert Opin Drug Metab Toxicol. 2009. PMID: 19442037 Review.
-
Organic anion transporting polypeptide (OATP)1B1 and OATP1B3 as important regulators of the pharmacokinetics of substrate drugs.Biol Pharm Bull. 2015;38(2):155-68. doi: 10.1248/bpb.b14-00767. Biol Pharm Bull. 2015. PMID: 25747975 Review.
Cited by
-
Pharmacogenetics Approach for the Improvement of COVID-19 Treatment.Viruses. 2021 Mar 5;13(3):413. doi: 10.3390/v13030413. Viruses. 2021. PMID: 33807592 Free PMC article. Review.
-
PharmVar GeneFocus: SLCO1B1.Clin Pharmacol Ther. 2023 Apr;113(4):782-793. doi: 10.1002/cpt.2705. Epub 2022 Jul 27. Clin Pharmacol Ther. 2023. PMID: 35797228 Free PMC article. Review.
-
The Impact of Scaling Factor Variability on Risk-Relevant Pharmacokinetic Outcomes in Children: A Case Study Using Bromodichloromethane (BDCM).Toxicol Sci. 2019 Feb 1;167(2):347-359. doi: 10.1093/toxsci/kfy236. Toxicol Sci. 2019. PMID: 30252107 Free PMC article.
-
Physiologically-Based Pharmacokinetic (PBPK) Modeling Providing Insights into Fentanyl Pharmacokinetics in Adults and Pediatric Patients.Pharmaceutics. 2020 Sep 23;12(10):908. doi: 10.3390/pharmaceutics12100908. Pharmaceutics. 2020. PMID: 32977559 Free PMC article.
-
Physiologically Based Pharmacokinetics Modeling in the Neonatal Population-Current Advances, Challenges, and Opportunities.Pharmaceutics. 2023 Nov 3;15(11):2579. doi: 10.3390/pharmaceutics15112579. Pharmaceutics. 2023. PMID: 38004559 Free PMC article. Review.
References
-
- Barrett JS, Della Casa Alberighi O, Läer S, Meibohm B. (2012) Physiologically based pharmacokinetic (PBPK) modeling in children. Clin Pharmacol Ther 92:40–49. - PubMed
-
- Bradford MM. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. - PubMed
-
- Brouwer KL, Aleksunes LM, Brandys B, Giacoia GP, Knipp G, Lukacova V, Meibohm B, Nigam SK, Rieder M, de Wildt SN, Pediatric Transporter Working Group (2015) Human ontogeny of drug transporters: review and recommendations of the Pediatric Transporter Working Group. Clin Pharmacol Ther 98:266–287. - PMC - PubMed
-
- Cheng X, Maher J, Chen C, Klaassen CD. (2005) Tissue distribution and ontogeny of mouse organic anion transporting polypeptides (OATPs). Drug Metab Dispos 33:1062–1073. - PubMed
-
- Cui Y, König J, Keppler D. (2001) Vectorial transport by double-transfected cells expressing the human uptake transporter SLC21A8 and the apical export pump ABCC2. Mol Pharmacol 60:934–943. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

