pEVL: A Linear Plasmid for Generating mRNA IVT Templates With Extended Encoded Poly(A) Sequences
- PMID: 27093168
- PMCID: PMC5014522
- DOI: 10.1038/mtna.2016.21
pEVL: A Linear Plasmid for Generating mRNA IVT Templates With Extended Encoded Poly(A) Sequences
Abstract
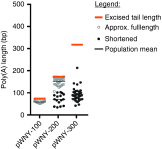
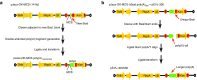
Increasing demand for large-scale synthesis of in vitro transcribed (IVT) mRNA is being driven by the increasing use of mRNA for transient gene expression in cell engineering and therapeutic applications. An important determinant of IVT mRNA potency is the 3' polyadenosine (poly(A)) tail, the length of which correlates with translational efficiency. However, present methods for generation of IVT mRNA rely on templates derived from circular plasmids or PCR products, in which homopolymeric tracts are unstable, thus limiting encoded poly(A) tail lengths to ~120 base pairs (bp). Here, we have developed a novel method for generation of extended poly(A) tracts using a previously described linear plasmid system, pJazz. We find that linear plasmids can successfully propagate poly(A) tracts up to ~500 bp in length for IVT mRNA production. We then modified pJazz by removing extraneous restriction sites, adding a T7 promoter sequence upstream from an extended multiple cloning site, and adding a unique type-IIS restriction site downstream from the encoded poly(A) tract to facilitate generation of IVT mRNA with precisely defined encoded poly(A) tracts and 3' termini. The resulting plasmid, designated pEVL, can be used to generate IVT mRNA with consistent defined lengths and terminal residue(s).
Figures
Similar articles
-
Recent Advances and Innovations in the Preparation and Purification of In Vitro-Transcribed-mRNA-Based Molecules.Pharmaceutics. 2023 Aug 23;15(9):2182. doi: 10.3390/pharmaceutics15092182. Pharmaceutics. 2023. PMID: 37765153 Free PMC article. Review.
-
A convenient method to generate and maintain poly(A)-encoding DNA sequences required for in vitro transcription of mRNA.Biotechniques. 2019 Jan;66(1):37-39. doi: 10.2144/btn-2018-0120. Biotechniques. 2019. PMID: 30730207
-
Poly A tail length analysis of in vitro transcribed mRNA by LC-MS.Anal Bioanal Chem. 2018 Feb;410(6):1667-1677. doi: 10.1007/s00216-017-0840-6. Epub 2018 Jan 8. Anal Bioanal Chem. 2018. PMID: 29313076
-
Purification of linearized template plasmid DNA decreases double-stranded RNA formation during IVT reaction.Front Mol Biosci. 2023 Sep 29;10:1248511. doi: 10.3389/fmolb.2023.1248511. eCollection 2023. Front Mol Biosci. 2023. PMID: 37842641 Free PMC article.
-
Emergence of synthetic mRNA: In vitro synthesis of mRNA and its applications in regenerative medicine.Biomaterials. 2018 Feb;156:172-193. doi: 10.1016/j.biomaterials.2017.11.034. Epub 2017 Nov 23. Biomaterials. 2018. PMID: 29197748 Review.
Cited by
-
Anion exchange HPLC monitoring of mRNA in vitro transcription reactions to support mRNA manufacturing process development.Front Mol Biosci. 2024 Mar 7;11:1250833. doi: 10.3389/fmolb.2024.1250833. eCollection 2024. Front Mol Biosci. 2024. PMID: 38516194 Free PMC article.
-
Modifications of mRNA vaccine structural elements for improving mRNA stability and translation efficiency.Mol Cell Toxicol. 2022;18(1):1-8. doi: 10.1007/s13273-021-00171-4. Epub 2021 Sep 20. Mol Cell Toxicol. 2022. PMID: 34567201 Free PMC article. Review.
-
Strategies for controlling the innate immune activity of conventional and self-amplifying mRNA therapeutics: Getting the message across.Adv Drug Deliv Rev. 2021 Sep;176:113900. doi: 10.1016/j.addr.2021.113900. Epub 2021 Jul 26. Adv Drug Deliv Rev. 2021. PMID: 34324884 Free PMC article. Review.
-
Modeling of MT. P495, an mRNA-based vaccine against the phosphate-binding protein PstS1 of Mycobacterium tuberculosis.Mol Divers. 2023 Aug;27(4):1613-1632. doi: 10.1007/s11030-022-10515-4. Epub 2022 Aug 25. Mol Divers. 2023. PMID: 36006502 Free PMC article.
-
Recent Advances and Innovations in the Preparation and Purification of In Vitro-Transcribed-mRNA-Based Molecules.Pharmaceutics. 2023 Aug 23;15(9):2182. doi: 10.3390/pharmaceutics15092182. Pharmaceutics. 2023. PMID: 37765153 Free PMC article. Review.
References
-
- Morgan, RA and Kakarla, S (2014). Genetic modification of T cells. Cancer J 20: 145–150. - PubMed
-
- Knudsen, ML, Ljungberg, K, Liljeström, P and Johansson, DX (2014). Intradermal electroporation of RNA. Methods Mol Biol 1121: 147–154. - PubMed
-
- Smits, E, Ponsaerts, P, Lenjou, M, Nijs, G, Van Bockstaele, DR, Berneman, ZN et al. (2004). RNA-based gene transfer for adult stem cells and T cells. Leukemia 18: 1898–1902. - PubMed
-
- Holtkamp, S, Kreiter, S, Selmi, A, Simon, P, Koslowski, M, Huber, C et al. (2006). Modification of antigen-encoding RNA increases stability, translational efficacy, and T-cell stimulatory capacity of dendritic cells. Blood 108: 4009–4017. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

