Shutoff of Host Gene Expression in Influenza A Virus and Herpesviruses: Similar Mechanisms and Common Themes
- PMID: 27092522
- PMCID: PMC4848596
- DOI: 10.3390/v8040102
Shutoff of Host Gene Expression in Influenza A Virus and Herpesviruses: Similar Mechanisms and Common Themes
Abstract
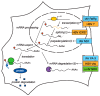
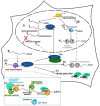
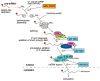
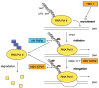
The ability to shut off host gene expression is a shared feature of many viral infections, and it is thought to promote viral replication by freeing host cell machinery and blocking immune responses. Despite the molecular differences between viruses, an emerging theme in the study of host shutoff is that divergent viruses use similar mechanisms to enact host shutoff. Moreover, even viruses that encode few proteins often have multiple mechanisms to affect host gene expression, and we are only starting to understand how these mechanisms are integrated. In this review we discuss the multiplicity of host shutoff mechanisms used by the orthomyxovirus influenza A virus and members of the alpha- and gamma-herpesvirus subfamilies. We highlight the surprising similarities in their mechanisms of host shutoff and discuss how the different mechanisms they use may play a coordinated role in gene regulation.
Keywords: Kaposi’s sarcoma-associated herpesvirus; RNA degradation; herpes simplex virus; herpesviruses; host shutoff; influenza A virus; transcription block.
Figures
Similar articles
-
Messenger RNA turnover and its regulation in herpesviral infection.Adv Virus Res. 2006;66:337-94. doi: 10.1016/S0065-3527(06)66007-7. Adv Virus Res. 2006. PMID: 16877064 Review.
-
Critical Role of the PA-X C-Terminal Domain of Influenza A Virus in Its Subcellular Localization and Shutoff Activity.J Virol. 2016 Jul 27;90(16):7131-7141. doi: 10.1128/JVI.00954-16. Print 2016 Aug 15. J Virol. 2016. PMID: 27226377 Free PMC article.
-
Host Shutoff in Influenza A Virus: Many Means to an End.Viruses. 2018 Sep 5;10(9):475. doi: 10.3390/v10090475. Viruses. 2018. PMID: 30189604 Free PMC article. Review.
-
Influenza A virus NS1 effector domain is required for PA-X-mediated host shutoff in infected cells.J Virol. 2024 May 14;98(5):e0190123. doi: 10.1128/jvi.01901-23. Epub 2024 Apr 17. J Virol. 2024. PMID: 38629840 Free PMC article.
-
Getting the message direct manipulation of host mRNA accumulation during gammaherpesvirus lytic infection.Adv Virus Res. 2010;78:1-42. doi: 10.1016/B978-0-12-385032-4.00001-X. Adv Virus Res. 2010. PMID: 21040830 Review.
Cited by
-
Mechanisms and consequences of mRNA destabilization during viral infections.Virol J. 2024 Feb 6;21(1):38. doi: 10.1186/s12985-024-02305-1. Virol J. 2024. PMID: 38321453 Free PMC article. Review.
-
Oncolytic Viruses as Antigen-Agnostic Cancer Vaccines.Cancer Cell. 2018 Apr 9;33(4):599-605. doi: 10.1016/j.ccell.2018.03.011. Cancer Cell. 2018. PMID: 29634947 Free PMC article. Review.
-
Viral Ubiquitin Ligase Stimulates Selective Host MicroRNA Expression by Targeting ZEB Transcriptional Repressors.Viruses. 2017 Aug 7;9(8):210. doi: 10.3390/v9080210. Viruses. 2017. PMID: 28783105 Free PMC article.
-
Functional and tissue enrichment analyses suggest that SARS-CoV-2 infection affects host metabolism and catabolism mediated by interference on host proteins.Braz J Microbiol. 2021 Sep;52(3):1151-1159. doi: 10.1007/s42770-021-00497-0. Epub 2021 May 6. Braz J Microbiol. 2021. PMID: 33956332 Free PMC article.
-
Host Innate Immune Response and Viral Immune Evasion During Alphaherpesvirus Infection.Curr Issues Mol Biol. 2021;42:635-686. doi: 10.21775/cimb.042.635. Epub 2021 Feb 28. Curr Issues Mol Biol. 2021. PMID: 33640867 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

