Age exacerbates the CCR2/5-mediated neuroinflammatory response to traumatic brain injury
- PMID: 27090212
- PMCID: PMC4835854
- DOI: 10.1186/s12974-016-0547-1
Age exacerbates the CCR2/5-mediated neuroinflammatory response to traumatic brain injury
Abstract
Background: Traumatic brain injury (TBI) is a major risk factor for the development of multiple neurodegenerative diseases, including Alzheimer's disease (AD) and numerous recent reports document the development of dementia after TBI. Age is a significant factor in both the risk of and the incidence of acquired brain injury. TBI-induced inflammatory response is associated with activation of brain resident microglia and accumulation of infiltrating monocytes, which plays a pivotal role in chronic neurodegeneration and loss of neurological function after TBI. Despite the extensive clinical evidence implicating neuroinflammation with the TBI-related sequelae, the specific role of these different myeloid cells and the influence of age on TBI-initiated innate immune response remain unknown and poorly studied.
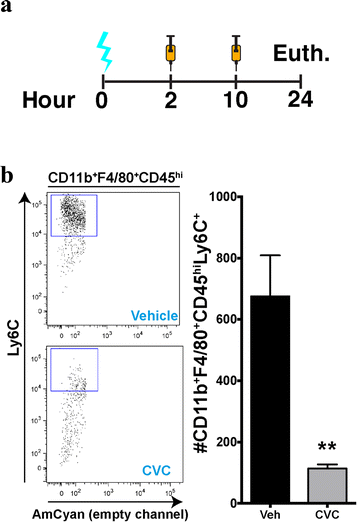
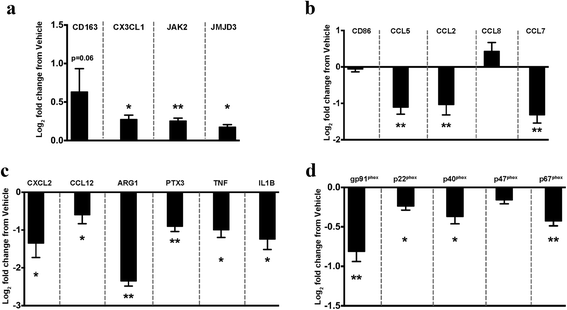
Methods: We used gene profiling and pathway analysis to define the effect of age on inflammatory response at the time of injury. The recruitment of peripheral CCR2(+) macrophages was delineated using the CX3CR1 (GFP/+) CCR2 (RFP/+) reporter mouse. These responses were examined in the context of CCR2/5 antagonism using cenicriviroc.
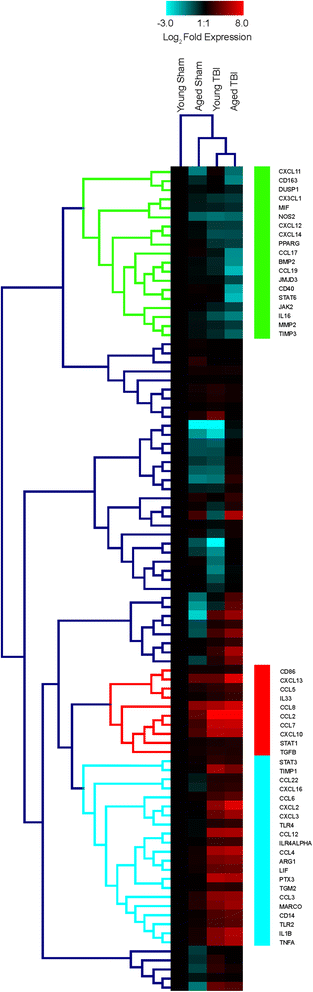
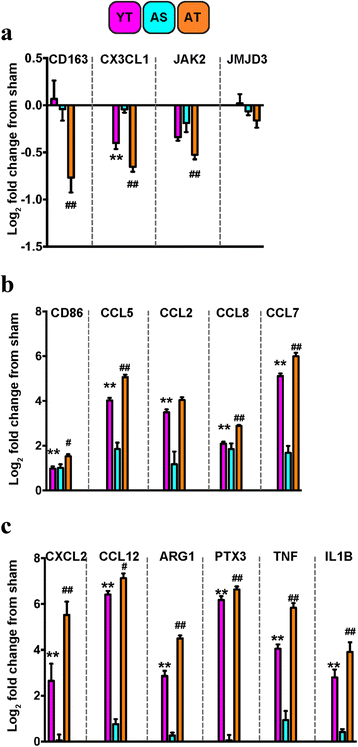
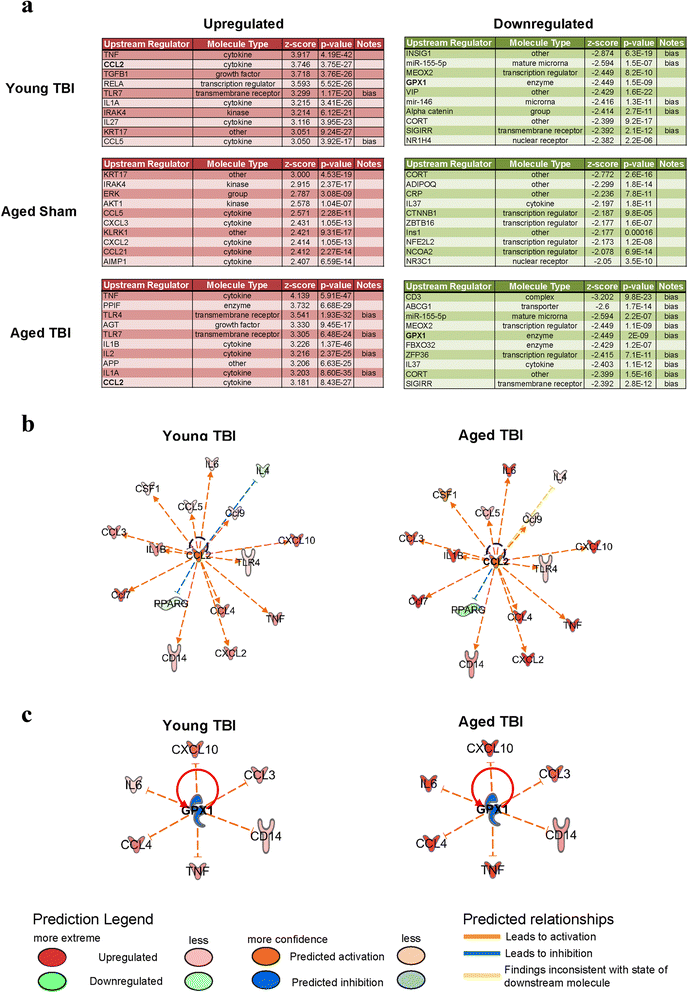
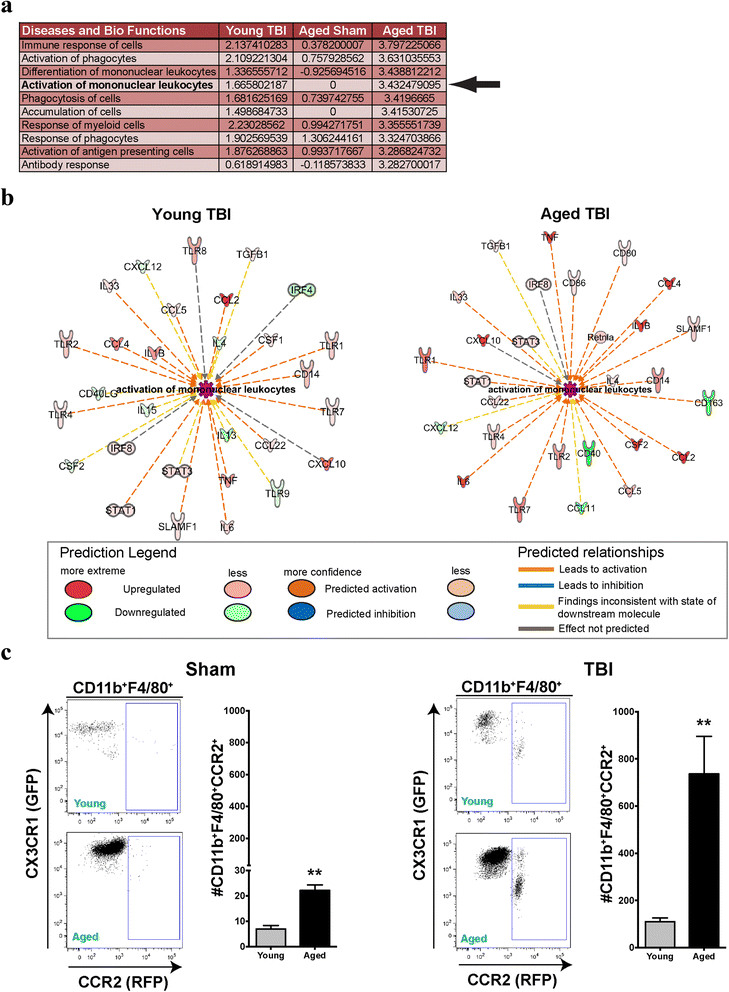
Results: Unsupervised gene clustering and pathway analysis revealed that age predisposes exacerbated inflammatory response related to the recruitment and activation of peripheral monocytes to the injured brain. Using a unique reporter animal model able to discriminate resident versus peripherally derived myeloid cells, we demonstrate that in the aged brain, there is an increased accumulation of peripherally derived CCR2(+) macrophages after TBI compared to young animals. Exaggerated recruitment of this population of cells was associated with an augmented inflammatory response in the aged TBI animals. Targeting this cellular response with cenicriviroc, a dual CCR2/5 antagonist, significantly ameliorated injury-induced sequelae in the aged TBI animals.
Conclusions: Importantly, these findings demonstrate that peripheral monocytes play a non-redundant and contributing role to the etiology of trauma-induced inflammatory sequelae in the aged brain.
Keywords: Aging; Antagonist; CCR2; Chemokine; Macrophage; Microglia; Neurotrauma.
Figures
Similar articles
-
Cellular players that shape evolving pathology and neurodegeneration following traumatic brain injury.Brain Behav Immun. 2018 Jul;71:9-17. doi: 10.1016/j.bbi.2018.03.033. Epub 2018 Mar 27. Brain Behav Immun. 2018. PMID: 29601944 Review.
-
CCR2 antagonism alters brain macrophage polarization and ameliorates cognitive dysfunction induced by traumatic brain injury.J Neurosci. 2015 Jan 14;35(2):748-60. doi: 10.1523/JNEUROSCI.2405-14.2015. J Neurosci. 2015. PMID: 25589768 Free PMC article.
-
Persistent Infiltration and Impaired Response of Peripherally-Derived Monocytes after Traumatic Brain Injury in the Aged Brain.Int J Mol Sci. 2018 May 30;19(6):1616. doi: 10.3390/ijms19061616. Int J Mol Sci. 2018. PMID: 29848996 Free PMC article.
-
Altered Neuroinflammation and Behavior after Traumatic Brain Injury in a Mouse Model of Alzheimer's Disease.J Neurotrauma. 2016 Apr 1;33(7):625-40. doi: 10.1089/neu.2015.3970. Epub 2015 Nov 23. J Neurotrauma. 2016. PMID: 26414955 Free PMC article.
-
The Inflammatory Continuum of Traumatic Brain Injury and Alzheimer's Disease.Front Immunol. 2018 Apr 9;9:672. doi: 10.3389/fimmu.2018.00672. eCollection 2018. Front Immunol. 2018. PMID: 29686672 Free PMC article. Review.
Cited by
-
Inhibition of the integrated stress response reverses cognitive deficits after traumatic brain injury.Proc Natl Acad Sci U S A. 2017 Aug 1;114(31):E6420-E6426. doi: 10.1073/pnas.1707661114. Epub 2017 Jul 10. Proc Natl Acad Sci U S A. 2017. PMID: 28696288 Free PMC article.
-
CC Chemokine Family Members' Modulation as a Novel Approach for Treating Central Nervous System and Peripheral Nervous System Injury-A Review of Clinical and Experimental Findings.Int J Mol Sci. 2024 Mar 28;25(7):3788. doi: 10.3390/ijms25073788. Int J Mol Sci. 2024. PMID: 38612597 Free PMC article. Review.
-
Microenvironmental Variations After Blood-Brain Barrier Breakdown in Traumatic Brain Injury.Front Mol Neurosci. 2021 Nov 26;14:750810. doi: 10.3389/fnmol.2021.750810. eCollection 2021. Front Mol Neurosci. 2021. PMID: 34899180 Free PMC article. Review.
-
Cell-Based Therapies for Traumatic Brain Injury: Therapeutic Treatments and Clinical Trials.Biomedicines. 2021 Jun 10;9(6):669. doi: 10.3390/biomedicines9060669. Biomedicines. 2021. PMID: 34200905 Free PMC article. Review.
-
Understanding Immune-Driven Brain Aging by Human Brain Organoid Microphysiological Analysis Platform.Adv Sci (Weinh). 2022 Sep;9(27):e2200475. doi: 10.1002/advs.202200475. Epub 2022 Jul 31. Adv Sci (Weinh). 2022. PMID: 35908805 Free PMC article.
References
-
- Stocchetti N, Paterno R, Citerio G, Beretta L, Colombo A: Traumatic brain injury in an aging population. J Neurotrauma. 2012; 29:1119-25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

