Purification and Biochemical Characterisation of Rabbit Calicivirus RNA-Dependent RNA Polymerases and Identification of Non-Nucleoside Inhibitors
- PMID: 27089358
- PMCID: PMC4848594
- DOI: 10.3390/v8040100
Purification and Biochemical Characterisation of Rabbit Calicivirus RNA-Dependent RNA Polymerases and Identification of Non-Nucleoside Inhibitors
Abstract
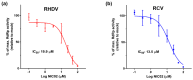
Rabbit haemorrhagic disease virus (RHDV) is a calicivirus that causes acute infections in both domestic and wild European rabbits (Oryctolagus cuniculus). The virus causes significant economic losses in rabbit farming and reduces wild rabbit populations. The recent emergence of RHDV variants capable of overcoming immunity to other strains emphasises the need to develop universally effective antivirals to enable quick responses during outbreaks until new vaccines become available. The RNA-dependent RNA polymerase (RdRp) is a primary target for the development of such antiviral drugs. In this study, we used cell-free in vitro assays to examine the biochemical characteristics of two rabbit calicivirus RdRps and the effects of several antivirals that were previously identified as human norovirus RdRp inhibitors. The non-nucleoside inhibitor NIC02 was identified as a potential scaffold for further drug development against rabbit caliciviruses. Our experiments revealed an unusually high temperature optimum (between 40 and 45 °C) for RdRps derived from both a pathogenic and a non-pathogenic rabbit calicivirus, possibly demonstrating an adaptation to a host with a physiological body temperature of more than 38 °C. Interestingly, the in vitro polymerase activity of the non-pathogenic calicivirus RdRp was at least two times higher than that of the RdRp of the highly virulent RHDV.
Keywords: RCV-A1; RHDV; antiviral agents; non-nucleoside inhibitors; polymerase.
Figures

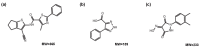


Similar articles
-
A Motif in the F Homomorph of Rabbit Haemorrhagic Disease Virus Polymerase Is Important for the Subcellular Localisation of the Protein and Its Ability to Induce Redistribution of Golgi Membranes.Viruses. 2017 Aug 1;9(8):202. doi: 10.3390/v9080202. Viruses. 2017. PMID: 28763035 Free PMC article.
-
Broad-spectrum non-nucleoside inhibitors for caliciviruses.Antiviral Res. 2017 Oct;146:65-75. doi: 10.1016/j.antiviral.2017.07.014. Epub 2017 Jul 27. Antiviral Res. 2017. PMID: 28757394
-
RNA-Dependent RNA Polymerases of Both Virulent and Benign Rabbit Caliciviruses Induce Striking Rearrangement of Golgi Membranes.PLoS One. 2017 Jan 10;12(1):e0169913. doi: 10.1371/journal.pone.0169913. eCollection 2017. PLoS One. 2017. PMID: 28072826 Free PMC article.
-
Structure(s), function(s), and inhibition of the RNA-dependent RNA polymerase of noroviruses.Virus Res. 2017 Apr 15;234:21-33. doi: 10.1016/j.virusres.2016.12.018. Epub 2016 Dec 29. Virus Res. 2017. PMID: 28041960 Free PMC article. Review.
-
Genetic variation and phylogenetic analysis of rabbit haemorrhagic disease virus (RHDV) strains.Acta Biochim Pol. 2012;59(4):459-65. Epub 2012 Dec 13. Acta Biochim Pol. 2012. PMID: 23240105 Review.
Cited by
-
Activity and cryo-EM structure of the polymerase domain of the human norovirus ProPol precursor.J Virol. 2024 Nov 19;98(11):e0119324. doi: 10.1128/jvi.01193-24. Epub 2024 Oct 30. J Virol. 2024. PMID: 39475276 Free PMC article.
-
A Motif in the F Homomorph of Rabbit Haemorrhagic Disease Virus Polymerase Is Important for the Subcellular Localisation of the Protein and Its Ability to Induce Redistribution of Golgi Membranes.Viruses. 2017 Aug 1;9(8):202. doi: 10.3390/v9080202. Viruses. 2017. PMID: 28763035 Free PMC article.
-
The Adenosine Analogue NITD008 has Potent Antiviral Activity against Human and Animal Caliciviruses.Viruses. 2019 May 30;11(6):496. doi: 10.3390/v11060496. Viruses. 2019. PMID: 31151251 Free PMC article.
-
Frequent intergenotypic recombination between the non-structural and structural genes is a major driver of epidemiological fitness in caliciviruses.Virus Evol. 2021 Sep 16;7(2):veab080. doi: 10.1093/ve/veab080. eCollection 2021. Virus Evol. 2021. PMID: 34754513 Free PMC article.
-
Potential Therapeutic Agents for Feline Calicivirus Infection.Viruses. 2018 Aug 16;10(8):433. doi: 10.3390/v10080433. Viruses. 2018. PMID: 30115859 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

