Ripple-triggered stimulation of the locus coeruleus during post-learning sleep disrupts ripple/spindle coupling and impairs memory consolidation
- PMID: 27084931
- PMCID: PMC4836638
- DOI: 10.1101/lm.040923.115
Ripple-triggered stimulation of the locus coeruleus during post-learning sleep disrupts ripple/spindle coupling and impairs memory consolidation
Abstract
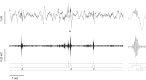
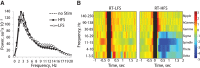
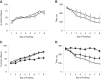
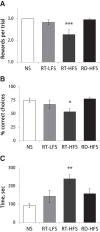
Experience-induced replay of neuronal ensembles occurs during hippocampal high-frequency oscillations, or ripples. Post-learning increase in ripple rate is predictive of memory recall, while ripple disruption impairs learning. Ripples may thus present a fundamental component of a neurophysiological mechanism of memory consolidation. In addition to system-level local and cross-regional interactions, a consolidation mechanism involves stabilization of memory representations at the synaptic level. Synaptic plasticity within experience-activated neuronal networks is facilitated by noradrenaline release from the axon terminals of the locus coeruleus (LC). Here, to better understand interactions between the system and synaptic mechanisms underlying "off-line" consolidation, we examined the effects of ripple-associated LC activation on hippocampal and cortical activity and on spatial memory. Rats were trained on a radial maze; after each daily learning session neural activity was monitored for 1 h via implanted electrode arrays. Immediately following "on-line" detection of ripple, a brief train of electrical pulses (0.05 mA) was applied to LC. Low-frequency (20 Hz) stimulation had no effect on spatial learning, while higher-frequency (100 Hz) trains transiently blocked generation of ripple-associated cortical spindles and caused a reference memory deficit. Suppression of synchronous ripple/spindle events appears to interfere with hippocampal-cortical communication, thereby reducing the efficiency of "off-line" memory consolidation.
© 2016 Novitskaya et al.; Published by Cold Spring Harbor Laboratory Press.
Figures
Similar articles
-
Abnormal Locus Coeruleus Sleep Activity Alters Sleep Signatures of Memory Consolidation and Impairs Place Cell Stability and Spatial Memory.Curr Biol. 2018 Nov 19;28(22):3599-3609.e4. doi: 10.1016/j.cub.2018.09.054. Epub 2018 Nov 1. Curr Biol. 2018. PMID: 30393040 Free PMC article.
-
Occurrence of Hippocampal Ripples is Associated with Activity Suppression in the Mediodorsal Thalamic Nucleus.J Neurosci. 2019 Jan 16;39(3):434-444. doi: 10.1523/JNEUROSCI.2107-18.2018. Epub 2018 Nov 20. J Neurosci. 2019. PMID: 30459228 Free PMC article.
-
Monosynaptic Hippocampal-Prefrontal Projections Contribute to Spatial Memory Consolidation in Mice.J Neurosci. 2019 Aug 28;39(35):6978-6991. doi: 10.1523/JNEUROSCI.2158-18.2019. Epub 2019 Jul 8. J Neurosci. 2019. PMID: 31285301 Free PMC article.
-
Slow oscillations orchestrating fast oscillations and memory consolidation.Prog Brain Res. 2011;193:93-110. doi: 10.1016/B978-0-444-53839-0.00007-7. Prog Brain Res. 2011. PMID: 21854958 Review.
-
[Hippocampal ripple oscillations (200 Hz) in mechanisms of memory consolidation].Usp Fiziol Nauk. 2002 Oct-Dec;33(4):34-42. Usp Fiziol Nauk. 2002. PMID: 12449805 Review. Russian.
Cited by
-
Causal Contribution of Awake Post-encoding Processes to Episodic Memory Consolidation.Curr Biol. 2020 Sep 21;30(18):3533-3543.e7. doi: 10.1016/j.cub.2020.06.063. Epub 2020 Jul 30. Curr Biol. 2020. PMID: 32735812 Free PMC article.
-
Trained-feature-specific offline learning by sleep in an orientation detection task.J Vis. 2019 Oct 1;19(12):12. doi: 10.1167/19.12.12. J Vis. 2019. PMID: 31622472 Free PMC article. Clinical Trial.
-
Real-time classification of experience-related ensemble spiking patterns for closed-loop applications.Elife. 2018 Oct 30;7:e36275. doi: 10.7554/eLife.36275. Elife. 2018. PMID: 30373716 Free PMC article.
-
Sleep fMRI with simultaneous electrophysiology at 9.4 T in male mice.Nat Commun. 2023 Mar 24;14(1):1651. doi: 10.1038/s41467-023-37352-9. Nat Commun. 2023. PMID: 36964161 Free PMC article.
-
Pathological changes of brain oscillations following ischemic stroke.J Cereb Blood Flow Metab. 2022 Oct;42(10):1753-1776. doi: 10.1177/0271678X221105677. Epub 2022 Jun 25. J Cereb Blood Flow Metab. 2022. PMID: 35754347 Free PMC article. Review.
References
-
- Bailey CH, Giustetto M, Huang Y-Y, Hawkins RD, Kandel ER. 2000. Is heterosynaptic modulation essential for stabilizing Hebbian plasticity and memory?. Nat Rev Neurosci 1: 11–20. - PubMed
-
- Battaglia FP, Benchenane K, Sirota A, Pennartz CM, Wiener SI. 2011. The hippocampus: hub of brain network communication for memory. Trends Cogn Sci 15: 310–318. - PubMed
-
- Behrens CJ, van den Boom LP, de Hoz L, Friedman A, Heinemann U. 2005. Induction of sharp wave-ripple complexes in vitro and reorganization of hippocampal networks. Nat Neurosci 8: 1560–1567. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
