A Central Role for Sympathetic Nerves in Herpes Stromal Keratitis in Mice
- PMID: 27070108
- PMCID: PMC4849540
- DOI: 10.1167/iovs.16-19183
A Central Role for Sympathetic Nerves in Herpes Stromal Keratitis in Mice
Abstract
Purpose: Herpes simplex virus type 1 (HSV-1) is a neurotrophic virus that can cause herpes stromal keratitis (HSK), a severe corneal inflammation that can lead to corneal scarring and blindness. This study identified neurologic changes that occur in HSV-1-infected corneas and related them to HSV-1-induced immunopathology.
Methods: Corneas of BALB/c and C57BL/6 mice were infected with HSV-1 strains that induce HSK. Changes in sensory nerves were identified by immunofluorescence staining of sensory and sympathetic nerves for substance P (SP) and tyrosine hydroxylase (TH), respectively, and confocal microscopic examination. Some mice received superior cervical ganglionectomy (SCGx) to eliminate sympathetic nerves from the cornea.
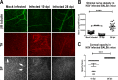
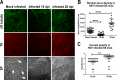
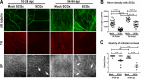
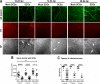
Results: Normal corneas exclusively expressed sensory nerves that entered the stroma as large nerve stalks, branched to form a plexus at the epithelial/stromal interface, and extended termini into the epithelium. These nerves completely retracted from the infected cornea and were replaced by sympathetic nerves that sprouted extensively to hyperinnervate the corneal stroma but failed to form a plexus or extend termini into the epithelium. The hyperinnervating nerves expressed the sympathetic nerve marker TH and their invasion was blocked by performing SCGx. Moreover, the corneal opacity and neovascularization that normally characterizes HSK in this mouse model were largely abrogated by SCGx. Sensory nerves reinnervated infected corneas following SCGx, reformed a nerve plexus, and extended termini into the epithelium resulting in recovery of corneal sensitivity.
Conclusions: Sympathetic nerves have a central role in HSK in mice, preventing reinnervation by sensory nerves and promoting severe and persistent corneal inflammation.
Figures

Similar articles
-
Pathogenesis of herpes simplex keratitis: The host cell response and ocular surface sequelae to infection and inflammation.Ocul Surf. 2019 Jan;17(1):40-49. doi: 10.1016/j.jtos.2018.10.002. Epub 2018 Oct 11. Ocul Surf. 2019. PMID: 30317007 Free PMC article. Review.
-
Sensory Nerve Retraction and Sympathetic Nerve Innervation Contribute to Immunopathology of Murine Recurrent Herpes Stromal Keratitis.Invest Ophthalmol Vis Sci. 2022 Feb 1;63(2):4. doi: 10.1167/iovs.63.2.4. Invest Ophthalmol Vis Sci. 2022. PMID: 35103749 Free PMC article.
-
Reversible nerve damage and corneal pathology in murine herpes simplex stromal keratitis.J Virol. 2014 Jul;88(14):7870-80. doi: 10.1128/JVI.01146-14. Epub 2014 Apr 30. J Virol. 2014. PMID: 24789786 Free PMC article.
-
Degeneration and regeneration of corneal nerves in response to HSV-1 infection.Invest Ophthalmol Vis Sci. 2015 Jan 13;56(2):1097-107. doi: 10.1167/iovs.14-15596. Invest Ophthalmol Vis Sci. 2015. PMID: 25587055 Free PMC article.
-
[Research progress of corneal neovascularization in herpes stromal keratitis].Zhonghua Yan Ke Za Zhi. 2019 Dec 11;55(12):956-960. doi: 10.3760/cma.j.issn.0412-4081.2019.12.017. Zhonghua Yan Ke Za Zhi. 2019. PMID: 31874509 Review. Chinese.
Cited by
-
Corneal esthesiometry and sub-basal nerves morphological changes in herpes simplex virus keratitis/uveitis patients.Int J Ophthalmol. 2019 Mar 18;12(3):407-411. doi: 10.18240/ijo.2019.03.09. eCollection 2019. Int J Ophthalmol. 2019. PMID: 30918808 Free PMC article.
-
Pathogenesis of herpes simplex keratitis: The host cell response and ocular surface sequelae to infection and inflammation.Ocul Surf. 2019 Jan;17(1):40-49. doi: 10.1016/j.jtos.2018.10.002. Epub 2018 Oct 11. Ocul Surf. 2019. PMID: 30317007 Free PMC article. Review.
-
Immunity and pain in the eye: focus on the ocular surface.Clin Exp Immunol. 2022 Apr 4;207(2):149-163. doi: 10.1093/cei/uxab032. Clin Exp Immunol. 2022. PMID: 35020868 Free PMC article. Review.
-
Harmol used for the treatment of herpes simplex virus induced keratitis.Virol J. 2024 May 27;21(1):118. doi: 10.1186/s12985-024-02384-0. Virol J. 2024. PMID: 38802860 Free PMC article.
-
Neuroanatomy and neurochemistry of rat cornea: Changes with age.Ocul Surf. 2021 Apr;20:86-94. doi: 10.1016/j.jtos.2020.11.005. Epub 2020 Dec 17. Ocul Surf. 2021. PMID: 33340717 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

