Antipsychotic Drugs Inhibit Platelet Aggregation via P2Y 1 and P2Y 12 Receptors
- PMID: 27069920
- PMCID: PMC4812202
- DOI: 10.1155/2016/2532371
Antipsychotic Drugs Inhibit Platelet Aggregation via P2Y 1 and P2Y 12 Receptors
Abstract
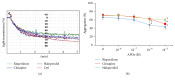
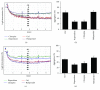
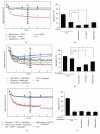
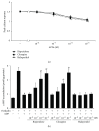
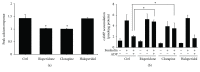
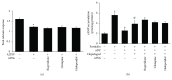
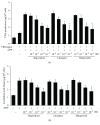
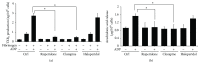
Antipsychotic drugs (APDs) used to treat clinical psychotic syndromes cause a variety of blood dyscrasias. APDs suppress the aggregation of platelets; however, the underlying mechanism remains unknown. We first analyzed platelet aggregation and clot formation in platelets treated with APDs, risperidone, clozapine, or haloperidol, using an aggregometer and rotational thromboelastometry (ROTEM). Our data indicated that platelet aggregation was inhibited, that clot formation time was increased, and that clot firmness was decreased in platelets pretreated with APDs. We also examined the role two major adenosine diphosphate (ADP) receptors, P2Y1 and P2Y12, play in ADP-mediated platelet activation and APD-mediated suppression of platelet aggregation. Our results show that P2Y1 receptor stimulation with ADP-induced calcium influx was inhibited by APDs in human and rats' platelets, as assessed by in vitro or ex vivo approach, respectively. In contrast, APDs, risperidone and clozapine, alleviated P2Y12-mediated cAMP suppression, and the release of thromboxane A2 and arachidonic acid by activated platelets decreased after APD treatment in human and rats' platelets. Our data demonstrate that each APD tested significantly suppressed platelet aggregation via different mechanisms.
Figures
Similar articles
-
Selective Inhibition of P2Y1 and P2Y12 Receptor Signal Pathways in Platelet Aggregation in Transgenic Cell Lines and Rats by Potassium 2-(1-Hydroxypentyl)-Benzoate, Puerarin and Salvianolic Acid B.CNS Neurosci Ther. 2024 Nov;30(11):e70089. doi: 10.1111/cns.70089. CNS Neurosci Ther. 2024. PMID: 39563013 Free PMC article.
-
Nucleoside triphosphates inhibit ADP, collagen, and epinephrine-induced platelet aggregation: role of P2Y₁ and P2Y₁₂ receptors.Thromb Res. 2013 Nov;132(5):548-57. doi: 10.1016/j.thromres.2013.08.021. Epub 2013 Sep 1. Thromb Res. 2013. PMID: 24071464
-
Effect of P2Y1 and P2Y12 genetic polymorphisms on the ADP-induced platelet aggregation in a Korean population.Thromb Res. 2013 Aug;132(2):221-6. doi: 10.1016/j.thromres.2013.06.020. Epub 2013 Jul 10. Thromb Res. 2013. PMID: 23849096
-
Scientific and therapeutic insights into the role of the platelet P2Y12 receptor in thrombosis.Curr Opin Hematol. 2003 Sep;10(5):333-8. doi: 10.1097/00062752-200309000-00002. Curr Opin Hematol. 2003. PMID: 12913786 Review.
-
Adenosine diphosphate receptors on blood platelets: potential new targets for antiplatelet therapy.Acta Biochim Pol. 2005;52(2):411-5. Epub 2005 May 15. Acta Biochim Pol. 2005. PMID: 15912207 Review.
Cited by
-
Selective Inhibition of P2Y1 and P2Y12 Receptor Signal Pathways in Platelet Aggregation in Transgenic Cell Lines and Rats by Potassium 2-(1-Hydroxypentyl)-Benzoate, Puerarin and Salvianolic Acid B.CNS Neurosci Ther. 2024 Nov;30(11):e70089. doi: 10.1111/cns.70089. CNS Neurosci Ther. 2024. PMID: 39563013 Free PMC article.
-
Endothelial dysfunction in neuroprogressive disorders-causes and suggested treatments.BMC Med. 2020 Oct 19;18(1):305. doi: 10.1186/s12916-020-01749-w. BMC Med. 2020. PMID: 33070778 Free PMC article. Review.
-
Safflower Extract Inhibits ADP-Induced Human Platelet Aggregation.Plants (Basel). 2021 Jun 11;10(6):1192. doi: 10.3390/plants10061192. Plants (Basel). 2021. PMID: 34208125 Free PMC article.
-
Role of ADP receptors on platelets in the growth of ovarian cancer.Blood. 2017 Sep 7;130(10):1235-1242. doi: 10.1182/blood-2017-02-769893. Epub 2017 Jul 5. Blood. 2017. PMID: 28679740 Free PMC article.
References
-
- Zhang X. Y., Zhou D. F., Cao L. Y., Zhang P. Y., Wu G. Y., Shen Y. C. Changes in serum interleukin-2, -6, and -8 levels before and during treatment with risperidone and haloperidol: relationship to outcome in schizophrenia. Journal of Clinical Psychiatry. 2004;65(7):940–947. doi: 10.4088/jcp.v65n0710. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

