Deguelin-induced blockade of PI3K/protein kinase B/MAP kinase signaling in zebrafish and breast cancer cell lines is mediated by down-regulation of fibroblast growth factor receptor 4 activity
- PMID: 27069628
- PMCID: PMC4804323
- DOI: 10.1002/prp2.212
Deguelin-induced blockade of PI3K/protein kinase B/MAP kinase signaling in zebrafish and breast cancer cell lines is mediated by down-regulation of fibroblast growth factor receptor 4 activity
Abstract
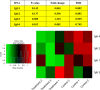
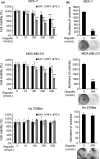
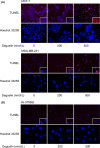
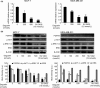
Deguelin, a natural component derived from leguminous plants, has been used as pesticide in some regions. Accumulating evidence show that deguelin has promising chemopreventive and therapeutic activities against cancer cells. This study shows that low concentrations of deguelin can lead to significant delay in zebrafish embryonic development through growth inhibition and induction of apoptosis. Furthermore, we identified fibroblast growth factor receptor 4 (FGFR4) as the putative target of deguelin. The candidate was initially identified by a microarray approach and then validated through in vitro experiments using hormone-responsive (MCF-7) and nonresponsive (MDA-MB-231) human breast cancer cell lines. The results show that deguelin suppressed cell proliferation and induced apoptosis in both cancer cell lines, but not in Hs 578Bst cells, by blocking PI3K/AKT and mitogen-activated protein kinases (MAPK) signaling. The FGFR4 mRNA and protein level also diminished in a dose-dependent manner. Interestingly, we found that forced FGFR4 overexpression attenuated deguelin-induced proliferative suppression and apoptotic cell death in both zebrafish and MCF-7 cell lines, p-AKT and p-ERK levels were restored upon FGFR4 overexpression. Taken together, our results strongly suggest that deguelin inhibition of PI3K/AKT and MAPK signaling in zebrafish and breast cancer cell lines is partially mediated through down-regulation of FGFR4 activity.
Keywords: Breast cancer; FGFR4; deguelin; zebrafish.
Figures


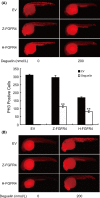

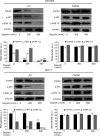
Similar articles
-
Effects of deguelin on the phosphatidylinositol 3-kinase/Akt pathway and apoptosis in premalignant human bronchial epithelial cells.J Natl Cancer Inst. 2003 Feb 19;95(4):291-302. doi: 10.1093/jnci/95.4.291. J Natl Cancer Inst. 2003. PMID: 12591985
-
[Effects of deguelin on proliferation and apoptosis of MCF-7 breast cancer cells by phosphatidylinositol 3-kinase/Akt signaling pathway].Zhong Xi Yi Jie He Xue Bao. 2011 May;9(5):533-8. doi: 10.3736/jcim20110511. Zhong Xi Yi Jie He Xue Bao. 2011. PMID: 21565140 Chinese.
-
Implication of AMP-activated protein kinase and Akt-regulated survivin in lung cancer chemopreventive activities of deguelin.Cancer Res. 2007 Dec 15;67(24):11630-9. doi: 10.1158/0008-5472.CAN-07-2401. Cancer Res. 2007. PMID: 18089792
-
Pharmacological basis and new insights of deguelin concerning its anticancer effects.Pharmacol Res. 2021 Dec;174:105935. doi: 10.1016/j.phrs.2021.105935. Epub 2021 Oct 10. Pharmacol Res. 2021. PMID: 34644595 Review.
-
Deguelin, a novel anti-tumorigenic agent targeting apoptosis, cell cycle arrest and anti-angiogenesis for cancer chemoprevention.Mol Clin Oncol. 2013 Mar;1(2):215-219. doi: 10.3892/mco.2012.36. Epub 2012 Oct 18. Mol Clin Oncol. 2013. PMID: 24649149 Free PMC article. Review.
Cited by
-
A novel approach for the identification of efficient combination therapies in primary human acute myeloid leukemia specimens.Blood Cancer J. 2017 Feb 17;7(2):e529. doi: 10.1038/bcj.2017.10. Blood Cancer J. 2017. PMID: 28211886 Free PMC article.
-
Deguelin Attenuates Allergic Airway Inflammation via Inhibition of NF-κb Pathway in Mice.Int J Biol Sci. 2017 Apr 8;13(4):492-504. doi: 10.7150/ijbs.17238. eCollection 2017. Int J Biol Sci. 2017. PMID: 28529457 Free PMC article.
-
Deguelin inhibits epithelial-to-mesenchymal transition and metastasis of human non-small cell lung cancer cells by regulating NIMA-related kinase 2.Thorac Cancer. 2017 Jul;8(4):320-327. doi: 10.1111/1759-7714.12444. Epub 2017 May 16. Thorac Cancer. 2017. PMID: 28509438 Free PMC article.
-
Deguelin exerts anticancer activity of human gastric cancer MGC-803 and MKN-45 cells in vitro.Int J Mol Med. 2018 Jun;41(6):3157-3166. doi: 10.3892/ijmm.2018.3532. Epub 2018 Mar 5. Int J Mol Med. 2018. PMID: 29512685 Free PMC article.
-
Toxicity testing of pesticides in zebrafish-a systematic review on chemicals and associated toxicological endpoints.Environ Sci Pollut Res Int. 2020 Apr;27(10):10185-10204. doi: 10.1007/s11356-020-07902-5. Epub 2020 Feb 15. Environ Sci Pollut Res Int. 2020. PMID: 32062774
References
-
- Agazie YM, Movilla N, Ischenko I, Hayman MJ (2003). The phosphotyrosine phosphatase SHP2 is a critical mediator of transformation induced by the oncogenic fibroblast growth factor receptor 3. Oncogene 22: 6909–6918. - PubMed
-
- Bange J, Prechtl D, Cheburkin Y, Specht K, Harbeck N, Schmitt M, et al. (2002). Cancer progression and tumor cell motility are associated with the FGFR4 Arg(388) allele. Cancer Res 62: 840–847. - PubMed
-
- Becker D, Lee PL, Rodeck U, Herlyn M (1992). Inhibition of the fibroblast growth factor receptor 1 (FGFR‐1) gene in human melanocytes and malignant melanomas leads to inhibition of proliferation and signs indicative of differentiation. Oncogene 7: 2303–2313. - PubMed
-
- Bortul R, Tazzari PL, Billi AM, Tabellini G, Mantovani I, Cappellini A, et al. (2005). Deguelin, A PI3K/AKT inhibitor, enhances chemosensitivity of leukaemia cells with an active PI3K/AKT pathway. Br J Haematol 129: 677–686. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

