Combination Pod-Intravaginal Ring Delivers Antiretroviral Agents for HIV Prophylaxis: Pharmacokinetic Evaluation in an Ovine Model
- PMID: 27067321
- PMCID: PMC4879417
- DOI: 10.1128/AAC.00391-16
Combination Pod-Intravaginal Ring Delivers Antiretroviral Agents for HIV Prophylaxis: Pharmacokinetic Evaluation in an Ovine Model
Abstract
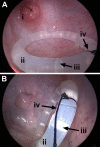
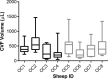
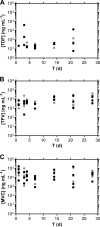
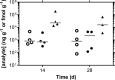
Preexposure prophylaxis (PrEP) against HIV using oral regimens based on the nucleoside reverse transcriptase inhibitor tenofovir disoproxil fumarate (TDF) has been effective to various degrees in multiple clinical trials, and the CCR5 receptor antagonist maraviroc (MVC) holds potential for complementary efficacy. The effectiveness of HIV PrEP is highly dependent on adherence. Incorporation of the TDF-MVC combination into intravaginal rings (IVRs) for sustained mucosal delivery could increase product adherence and efficacy compared with oral and vaginal gel formulations. A novel pod-IVR technology capable of delivering multiple drugs is described. The pharmacokinetics and preliminary local safety characteristics of a novel pod-IVR delivering a combination of TDF and MVC were evaluated in the ovine model. The device exhibited sustained release at controlled rates over the 28-day study and maintained steady-state drug levels in cervicovaginal fluids (CVFs). Dilution of CVFs during lavage sample collection was measured by ion chromatography using an inert tracer, allowing corrected drug concentrations to be measured for the first time. Median, steady-state drug levels in vaginal tissue homogenate were as follows: for tenofovir (TFV; in vivo hydrolysis product of TDF), 7.3 × 10(2) ng g(-1) (interquartile range [IQR], 3.0 × 10(2), 4.0 × 10(3)); for TFV diphosphate (TFV-DP; active metabolite of TFV), 1.8 × 10(4) fmol g(-1) (IQR, 1.5 × 10(4), 4.8 × 10(4)); and for MVC, 8.2 × 10(2) ng g(-1) (IQR, 4.7 × 10(2), 2.0 × 10(3)). No adverse events were observed. These findings, together with previous pod-IVR studies, have allowed several lead candidates to advance into clinical evaluation.
Trial registration: ClinicalTrials.gov NCT02431273.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures

Similar articles
-
Safety and pharmacokinetics of single, dual, and triple antiretroviral drug formulations delivered by pod-intravaginal rings designed for HIV-1 prevention: A Phase I trial.PLoS Med. 2018 Sep 28;15(9):e1002655. doi: 10.1371/journal.pmed.1002655. eCollection 2018 Sep. PLoS Med. 2018. PMID: 30265679 Free PMC article. Clinical Trial.
-
Pharmacokinetics and preliminary safety study of pod-intravaginal rings delivering antiretroviral combinations for HIV prophylaxis in a macaque model.Antimicrob Agents Chemother. 2014 Sep;58(9):5125-35. doi: 10.1128/AAC.02871-14. Epub 2014 Jun 16. Antimicrob Agents Chemother. 2014. PMID: 24936594 Free PMC article.
-
A phase 1 randomized placebo-controlled safety and pharmacokinetic trial of a tenofovir disoproxil fumarate vaginal ring.AIDS. 2016 Mar 13;30(5):743-51. doi: 10.1097/QAD.0000000000000979. AIDS. 2016. PMID: 26605514 Free PMC article. Clinical Trial.
-
The Vaginal Microbiome and its Potential to Impact Efficacy of HIV Pre-exposure Prophylaxis for Women.Curr HIV/AIDS Rep. 2017 Oct;14(5):153-160. doi: 10.1007/s11904-017-0362-z. Curr HIV/AIDS Rep. 2017. PMID: 28812207 Free PMC article. Review.
-
The future of pre-exposure prophylaxis (PrEP) for human immunodeficiency virus (HIV) infection.Expert Rev Anti Infect Ther. 2017 May;15(5):467-481. doi: 10.1080/14787210.2017.1309292. Epub 2017 Apr 4. Expert Rev Anti Infect Ther. 2017. PMID: 28322067 Review.
Cited by
-
Transcutaneously refillable nanofluidic implant achieves sustained level of tenofovir diphosphate for HIV pre-exposure prophylaxis.J Control Release. 2018 Sep 28;286:315-325. doi: 10.1016/j.jconrel.2018.08.010. Epub 2018 Aug 6. J Control Release. 2018. PMID: 30092254 Free PMC article.
-
Vaginal rings with exposed cores for sustained delivery of the HIV CCR5 inhibitor 5P12-RANTES.J Control Release. 2019 Mar 28;298:1-11. doi: 10.1016/j.jconrel.2019.02.003. Epub 2019 Feb 4. J Control Release. 2019. PMID: 30731150 Free PMC article.
-
Historical development of vaginal microbicides to prevent sexual transmission of HIV in women: from past failures to future hopes.Drug Des Devel Ther. 2017 Jun 15;11:1767-1787. doi: 10.2147/DDDT.S133170. eCollection 2017. Drug Des Devel Ther. 2017. PMID: 28670111 Free PMC article. Review.
-
Phase I trial of pod-intravaginal rings delivering antiretroviral agents for HIV-1 prevention: Rectal drug exposure from vaginal dosing with tenofovir disoproxil fumarate, emtricitabine, and maraviroc.PLoS One. 2018 Aug 22;13(8):e0201952. doi: 10.1371/journal.pone.0201952. eCollection 2018. PLoS One. 2018. PMID: 30133534 Free PMC article. Clinical Trial.
-
Design and Characterization of a Novel Series of Geometrically Complex Intravaginal Rings with Digital Light Synthesis.Adv Mater Technol. 2020 Aug;5(8):2000261. doi: 10.1002/admt.202000261. Epub 2020 Jun 23. Adv Mater Technol. 2020. PMID: 33072856 Free PMC article.
References
-
- Abdool Karim Q, Abdool Karim SS, Frohlich JA, Grobler AC, Baxter C, Mansoor LE, Kharsany ABM, Sibeko S, Mlisana KP, Omar Z, Gengiah TN, Maarschalk S, Arulappan N, Mlotshwa M, Morris L, Taylor D. 2010. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science 329:1168–1174. doi:10.1126/science.1193748. - DOI - PMC - PubMed
-
- Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, Goicochea P, Casapia M, Guanira-Carranza JV, Ramirez-Cardich ME, Montoya-Herrera O, Fernandez T, Veloso VG, Buchbinder SP, Chariyalertsak S, Schechter M, Bekker LG, Mayer KH, Kallas EG, Amico KR, Mulligan K, Bushman LR, Hance RJ, Ganoza C, Defechereux P, Postle B, Wang FR, McConnell JJ, Zheng JH, Lee J, Rooney JF, Jaffe HS, Martinez AI, Burns DN, Glidden DV. 2010. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 363:2587–2599. doi:10.1056/NEJMoa1011205. - DOI - PMC - PubMed
-
- Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, Tappero JW, Bukusi EA, Cohen CR, Katabira E, Ronald A, Tumwesigye E, Were E, Fife KH, Kiarie J, Farquhar C, John-Stewart G, Kakia A, Odoyo J, Mucunguzi A, Nakku-Joloba E, Twesigye R, Ngure K, Apaka C, Tamooh H, Gabona F, Mujugira A, Panteleeff D, Thomas KK, Kidoguchi L, Krows M, Revall J, Morrison S, Haugen H, Emmanuel-Ogier M, Ondrejcek L, Coombs RW, Frenkel L, Hendrix C, Bumpus NN, Bangsberg D, Haberer JE, Stevens WS, Lingappa JR, Celum C; Partners PrEP Study Team. 2012. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med 367:399–410. doi:10.1056/NEJMoa1108524. - DOI - PMC - PubMed
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

