Regulatory Networks in Pollen Development under Cold Stress
- PMID: 27066044
- PMCID: PMC4814731
- DOI: 10.3389/fpls.2016.00402
Regulatory Networks in Pollen Development under Cold Stress
Abstract
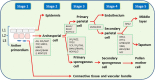
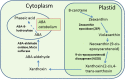
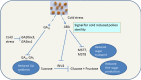
Cold stress modifies anthers' metabolic pathways to induce pollen sterility. Cold-tolerant plants, unlike the susceptible ones, produce high proportion of viable pollen. Anthers in susceptible plants, when exposed to cold stress, increase abscisic acid (ABA) metabolism and reduce ABA catabolism. Increased ABA negatively regulates expression of tapetum cell wall bound invertase and monosaccharide transport genes resulting in distorted carbohydrate pool in anther. Cold-stress also reduces endogenous levels of the bioactive gibberellins (GAs), GA4 and GA7, in susceptible anthers by repression of the GA biosynthesis genes. Here, we discuss recent findings on mechanisms of cold susceptibility in anthers which determine pollen sterility. We also discuss differences in regulatory pathways between cold-stressed anthers of susceptible and tolerant plants that decide pollen sterility or viability.
Keywords: abscisic acid signaling; anther; bioactive gibberellins; cold stress; pollen development; pollen sterility; sugar metabolism.
Figures

Similar articles
-
ABA regulates apoplastic sugar transport and is a potential signal for cold-induced pollen sterility in rice.Plant Cell Physiol. 2007 Sep;48(9):1319-30. doi: 10.1093/pcp/pcm100. Epub 2007 Aug 10. Plant Cell Physiol. 2007. PMID: 17693452
-
Control of abscisic acid catabolism and abscisic acid homeostasis is important for reproductive stage stress tolerance in cereals.Plant Physiol. 2011 Jun;156(2):647-62. doi: 10.1104/pp.111.176164. Epub 2011 Apr 18. Plant Physiol. 2011. PMID: 21502188 Free PMC article.
-
Cold Stress Response Mechanisms in Anther Development.Int J Mol Sci. 2022 Dec 20;24(1):30. doi: 10.3390/ijms24010030. Int J Mol Sci. 2022. PMID: 36613473 Free PMC article. Review.
-
Cold stress alters transcription in meiotic anthers of cold tolerant chickpea (Cicer arietinum L.).BMC Res Notes. 2014 Oct 11;7:717. doi: 10.1186/1756-0500-7-717. BMC Res Notes. 2014. PMID: 25306382 Free PMC article.
-
Crop Pollen Development under Drought: From the Phenotype to the Mechanism.Int J Mol Sci. 2019 Mar 28;20(7):1550. doi: 10.3390/ijms20071550. Int J Mol Sci. 2019. PMID: 30925673 Free PMC article. Review.
Cited by
-
Abscisic Acid-Enemy or Savior in the Response of Cereals to Abiotic and Biotic Stresses?Int J Mol Sci. 2020 Jun 29;21(13):4607. doi: 10.3390/ijms21134607. Int J Mol Sci. 2020. PMID: 32610484 Free PMC article. Review.
-
ZmRPN1 confers quantitative variation in pollen number and boosts hybrid seed production in maize.Plant Biotechnol J. 2023 Oct;21(10):1978-1989. doi: 10.1111/pbi.14105. Epub 2023 Jun 21. Plant Biotechnol J. 2023. PMID: 37341033 Free PMC article.
-
Role of exogenous abscisic acid in freezing tolerance of mangrove Kandelia obovata under natural frost condition at near 32°N.BMC Plant Biol. 2022 Dec 19;22(1):593. doi: 10.1186/s12870-022-03990-2. BMC Plant Biol. 2022. PMID: 36529723 Free PMC article.
-
Integrated Isoform Sequencing and Dynamic Transcriptome Analysis Reveals Diverse Transcripts Responsible for Low Temperature Stress at Anther Meiosis Stage in Rice.Front Plant Sci. 2021 Dec 17;12:795834. doi: 10.3389/fpls.2021.795834. eCollection 2021. Front Plant Sci. 2021. PMID: 34975985 Free PMC article.
-
Impedance Flow Cytometry: A Novel Technique in Pollen Analysis.PLoS One. 2016 Nov 10;11(11):e0165531. doi: 10.1371/journal.pone.0165531. eCollection 2016. PLoS One. 2016. PMID: 27832091 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

