Hemodynamics
- PMID: 27065172
- PMCID: PMC4958049
- DOI: 10.1002/cphy.c150038
Hemodynamics
Abstract
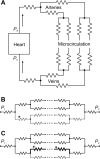
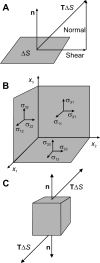
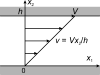
A review is presented of the physical principles governing the distribution of blood flow and blood pressure in the vascular system. The main factors involved are the pulsatile driving pressure generated by the heart, the flow characteristics of blood, and the geometric structure and mechanical properties of the vessels. The relationship between driving pressure and flow in a given vessel can be understood by considering the viscous and inertial forces acting on the blood. Depending on the vessel diameter and other physical parameters, a wide variety of flow phenomena can occur. In large arteries, the propagation of the pressure pulse depends on the elastic properties of the artery walls. In the microcirculation, the fact that blood is a suspension of cells strongly influences its flow properties and leads to a nonuniform distribution of hematocrit among microvessels. The forces acting on vessel walls include shear stress resulting from blood flow and circumferential stress resulting from blood pressure. Biological responses to these forces are important in the control of blood flow and the structural remodeling of vessels, and also play a role in major disease processes including hypertension and atherosclerosis. Consideration of hemodynamics is essential for a comprehensive understanding of the functioning of the circulatory system.
Copyright © 2016 John Wiley & Sons, Inc.
Figures

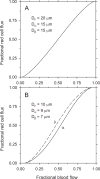
Similar articles
-
Remodeling of blood vessels: responses of diameter and wall thickness to hemodynamic and metabolic stimuli.Hypertension. 2005 Oct;46(4):725-31. doi: 10.1161/01.HYP.0000184428.16429.be. Epub 2005 Sep 19. Hypertension. 2005. PMID: 16172421
-
Reduced-order modeling of hemodynamics across macroscopic through mesoscopic circulation scales.Int J Numer Method Biomed Eng. 2019 Dec;35(12):e3274. doi: 10.1002/cnm.3274. Epub 2019 Nov 19. Int J Numer Method Biomed Eng. 2019. PMID: 31680447
-
Regulation of coronary blood flow during exercise.Physiol Rev. 2008 Jul;88(3):1009-86. doi: 10.1152/physrev.00045.2006. Physiol Rev. 2008. PMID: 18626066 Review.
-
Dynamic viscous flow in distensible vessels of skeletal muscle microcirculation: application to pressure and flow transients.Biorheology. 1989;26(2):215-27. doi: 10.3233/bir-1989-26209. Biorheology. 1989. PMID: 2605329
-
Structural adaptation of microvascular networks and development of hypertension.Microcirculation. 2002;9(4):305-14. doi: 10.1038/sj.mn.7800144. Microcirculation. 2002. PMID: 12152106 Review.
Cited by
-
Computational framework for the generation of one-dimensional vascular models accounting for uncertainty in networks extracted from medical images.ArXiv [Preprint]. 2024 May 9:arXiv:2309.08779v3. ArXiv. 2024. Update in: J Physiol. 2024 Aug;602(16):3929-3954. doi: 10.1113/JP286193. PMID: 38313199 Free PMC article. Updated. Preprint.
-
Computational simulation-derived hemodynamic and biomechanical properties of the pulmonary arterial tree early in the course of ventricular septal defects.Biomech Model Mechanobiol. 2021 Dec;20(6):2471-2489. doi: 10.1007/s10237-021-01519-4. Epub 2021 Sep 28. Biomech Model Mechanobiol. 2021. PMID: 34585299 Free PMC article.
-
The coronary capillary bed and its role in blood flow and oxygen delivery: A review.Anat Rec (Hoboken). 2022 Nov;305(11):3199-3211. doi: 10.1002/ar.24951. Epub 2022 May 25. Anat Rec (Hoboken). 2022. PMID: 35521832 Free PMC article. Review.
-
When it doesn't run in the blood(vessels) - events involved in vascular disorders.Biomed J. 2023 Apr;46(2):100591. doi: 10.1016/j.bj.2023.03.004. Epub 2023 Apr 13. Biomed J. 2023. PMID: 37059363 Free PMC article.
-
Optical blood pressure estimation with photoplethysmography and FFT-based neural networks.Biomed Opt Express. 2016 Jul 12;7(8):3007-20. doi: 10.1364/BOE.7.003007. eCollection 2016 Aug 1. Biomed Opt Express. 2016. PMID: 27570693 Free PMC article.
References
-
- Asmar R, Rudnichi A, Blacher J, London GM, Safar ME. Pulse pressure and aortic pulse wave are markers of cardiovascular risk in hypertensive populations. Am J Hypertens. 2001;14:91–97. - PubMed
-
- Avolio AP, Van Bortel LM, Boutouyrie P, Cockcroft JR, McEniery CM, Protogerou AD, Roman MJ, Safar ME, Segers P, Smulyan H. Role of pulse pressure amplification in arterial hypertension: experts’ opinion and review of the data. Hypertension. 2009;54:375–383. - PubMed
-
- Batchelor GK. An Introduction to Fluid Mechanics. Cambridge University Press; 1967.
-
- Bertram CD, Butcher KS. Possible sources of discrepancy between sphygmomanometer cuff pressure and blood pressure quantified in a collapsible-tube analogue. J Biomech Eng. 1992;114:68–77. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

