Rab43 regulates the sorting of a subset of membrane protein cargo through the medial Golgi
- PMID: 27053659
- PMCID: PMC4884073
- DOI: 10.1091/mbc.E15-03-0123
Rab43 regulates the sorting of a subset of membrane protein cargo through the medial Golgi
Abstract
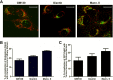
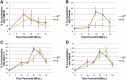
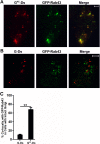
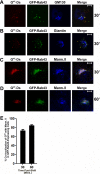
To evaluate the role of cytoplasmic domains of membrane-spanning proteins in directing trafficking through the secretory pathway, we generated fluorescently tagged VSV G tsO45 with either the native G tail (G) or a cytoplasmic tail derived from the chicken AE1-4 anion exchanger (G(AE)). We previously showed that these two proteins progressed through the Golgi with distinct kinetics. To investigate the basis for the differential sorting of G and G(AE), we analyzed the role of several Golgi-associated small GTP-binding proteins and found that Rab43 differentially regulated their transport through the Golgi. We show that the expression of GFP-Rab43 arrested the anterograde transport of G(AE) in a Rab43-positive medial Golgi compartment. GFP-Rab43 expression also inhibited the acquisition of endoH-resistant sugars and the surface delivery of G(AE), as well as the surface delivery of the AE1-4 anion exchanger. In contrast, GFP-Rab43 expression did not affect the glycosylation or surface delivery of G. Unexpectedly, down-regulation of endogenous Rab43 using small interfering RNA resulted in an increase in the accumulation of G(AE) on the cell surface while having minimal effect on the surface levels of G. Our data demonstrate that Rab43 regulates the sorting of a subset of membrane-spanning cargo as they progress through the medial Golgi.
© 2016 Cox et al. This article is distributed by The American Society for Cell Biology under license from the author(s). Two months after publication it is available to the public under an Attribution–Noncommercial–Share Alike 3.0 Unported Creative Commons License (http://creativecommons.org/licenses/by-nc-sa/3.0).
Figures
Similar articles
-
The GTPase Rab43 Controls the Anterograde ER-Golgi Trafficking and Sorting of GPCRs.Cell Rep. 2017 Oct 24;21(4):1089-1101. doi: 10.1016/j.celrep.2017.10.011. Cell Rep. 2017. PMID: 29069590 Free PMC article.
-
Kinetically distinct sorting pathways through the Golgi exhibit different requirements for Arf1.Traffic. 2015 Mar;16(3):267-83. doi: 10.1111/tra.12248. Epub 2015 Jan 12. Traffic. 2015. PMID: 25470762
-
Rab43 GTPase directs postsynaptic trafficking and neuron-specific sorting of G protein-coupled receptors.J Biol Chem. 2021 Jan-Jun;296:100517. doi: 10.1016/j.jbc.2021.100517. Epub 2021 Mar 4. J Biol Chem. 2021. PMID: 33676895 Free PMC article.
-
Are Rab proteins the link between Golgi organization and membrane trafficking?Cell Mol Life Sci. 2012 Dec;69(24):4093-106. doi: 10.1007/s00018-012-1021-6. Epub 2012 May 13. Cell Mol Life Sci. 2012. PMID: 22581368 Free PMC article. Review.
-
Golgi trafficking defects in postnatal microcephaly: The evidence for "Golgipathies".Prog Neurobiol. 2017 Jun;153:46-63. doi: 10.1016/j.pneurobio.2017.03.007. Epub 2017 Apr 2. Prog Neurobiol. 2017. PMID: 28377289 Review.
Cited by
-
The GTPase Rab43 Controls the Anterograde ER-Golgi Trafficking and Sorting of GPCRs.Cell Rep. 2017 Oct 24;21(4):1089-1101. doi: 10.1016/j.celrep.2017.10.011. Cell Rep. 2017. PMID: 29069590 Free PMC article.
-
Consequences of Rab GTPase dysfunction in genetic or acquired human diseases.Small GTPases. 2018 Mar 4;9(1-2):158-181. doi: 10.1080/21541248.2017.1397833. Epub 2017 Dec 28. Small GTPases. 2018. PMID: 29239692 Free PMC article. Review.
-
RBFOX2/GOLIM4 Splicing Axis Activates Vesicular Transport Pathway to Promote Nasopharyngeal Carcinogenesis.Adv Sci (Weinh). 2021 Aug;8(16):e2004852. doi: 10.1002/advs.202004852. Epub 2021 Jun 28. Adv Sci (Weinh). 2021. PMID: 34180133 Free PMC article.
-
Rab proteins as major determinants of the Golgi complex structure.Small GTPases. 2018 Mar 4;9(1-2):66-75. doi: 10.1080/21541248.2017.1384087. Epub 2018 Jan 29. Small GTPases. 2018. PMID: 29099310 Free PMC article. Review.
-
A germline mutation in Rab43 gene identified from a cancer family predisposes to a hereditary liver-colon cancer syndrome.BMC Cancer. 2019 Jun 21;19(1):613. doi: 10.1186/s12885-019-5845-4. BMC Cancer. 2019. PMID: 31226964 Free PMC article.
References
-
- Balch WE, Kahn RA, Schwaninger R. ADP-ribosylation factor is required for vesicular trafficking between the endoplasmic reticulum and the cis-Golgi compartment. J Biol Chem. 1992;267:13053–13061. - PubMed
-
- Barr FA. Rab GTPase function in Golgi trafficking. Semin Cell Dev Biol. 2009;20:780–783. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

