HIV-1 Tat Protein Activates both the MyD88 and TRIF Pathways To Induce Tumor Necrosis Factor Alpha and Interleukin-10 in Human Monocytes
- PMID: 27053552
- PMCID: PMC4907244
- DOI: 10.1128/JVI.00262-16
HIV-1 Tat Protein Activates both the MyD88 and TRIF Pathways To Induce Tumor Necrosis Factor Alpha and Interleukin-10 in Human Monocytes
Abstract
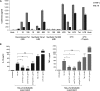
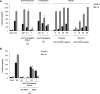
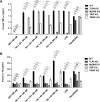
In this study, we show that the HIV-1 Tat protein interacts with rapid kinetics to engage the Toll-like receptor 4 (TLR4) pathway, leading to the production of proinflammatory and anti-inflammatory cytokines. The pretreatment of human monocytes with Tat protein for 10 to 30 min suffices to irreversibly engage the activation of the TLR4 pathway, leading to the production of tumor necrosis factor alpha (TNF-α) and interleukin-10 (IL-10), two cytokines strongly implicated in the chronic activation and dysregulation of the immune system during HIV-1 infection. Therefore, this study analyzed whether the HIV-1 Tat protein is able to activate these two pathways separately or simultaneously. Using three complementary approaches, including mice deficient in the MyD88, TIRAP/MAL, or TRIF adaptor, biochemical analysis, and the use of specific small interfering RNAs (siRNAs), we demonstrated (i) that Tat was able to activate both the MyD88 and TRIF pathways, (ii) the capacity of Tat to induce TIRAP/MAL degradation, (iii) the crucial role of the MyD88 pathway in the production of Tat-induced TNF-α and IL-10, (iv) a reduction but not abrogation of IL-10 and TNF-α by Tat-stimulated macrophages from mice deficient in TIRAP/MAL, and (v) the crucial role of the TRIF pathway in Tat-induced IL-10 production. Further, we showed that downstream of the MyD88 and TRIF pathways, the Tat protein activated the protein kinase C (PKC) βII isoform, the mitogen-activated protein (MAP) kinases p38 and extracellular signal-regulated kinase 1/2 (ERK1/2), and NF-κB in a TLR4-dependent manner. Collectively, our data show that by recruiting the TLR4 pathway with rapid kinetics, the HIV-1 Tat protein leads to the engagement of both the MyD88 and TRIF pathways and to the activation of PKC, MAP kinase, and NF-κB signaling to induce the production of TNF-α and IL-10.
Importance: In this study, we demonstrate that by recruiting the TLR4 pathway with rapid kinetics, the HIV-1 Tat protein leads to the engagement of both the MyD88 and TRIF pathways and to the activation of PKC-βII, MAP kinase, and NF-κB signaling to induce the production of TNF-α and IL-10, two cytokines strongly implicated in the chronic activation and dysregulation of the immune system during HIV-1 infection. Thus, it may be interesting to target Tat as a pathogenic factor early after HIV-1 infection. This could be achieved either by vaccination approaches including Tat as an immunogen in potential candidate vaccines or by developing molecules capable of neutralizing the effect of the Tat protein.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures



Similar articles
-
The mechanism of LPS-induced HIV type I activation in transgenic mouse macrophages.Int Immunol. 2010 Jun;22(6):469-78. doi: 10.1093/intimm/dxq032. Epub 2010 May 26. Int Immunol. 2010. PMID: 20504885
-
HIV-1 Tat protein induces TNF-alpha and IL-10 production by human macrophages: differential implication of PKC-betaII and -delta isozymes and MAP kinases ERK1/2 and p38.Cell Immunol. 2008;254(1):46-55. doi: 10.1016/j.cellimm.2008.06.011. Epub 2008 Aug 8. Cell Immunol. 2008. PMID: 18692180
-
Melatonin modulates TLR4-mediated inflammatory genes through MyD88- and TRIF-dependent signaling pathways in lipopolysaccharide-stimulated RAW264.7 cells.J Pineal Res. 2012 Nov;53(4):325-34. doi: 10.1111/j.1600-079X.2012.01002.x. Epub 2012 Apr 27. J Pineal Res. 2012. PMID: 22537289
-
Modulation of Toll-interleukin 1 receptor mediated signaling.J Mol Med (Berl). 2005 Apr;83(4):258-66. doi: 10.1007/s00109-004-0622-4. Epub 2005 Jan 21. J Mol Med (Berl). 2005. PMID: 15662540 Review.
-
Microbial recognition by Toll-like receptors.J Dermatol Sci. 2004 Apr;34(2):73-82. doi: 10.1016/j.jdermsci.2003.10.002. J Dermatol Sci. 2004. PMID: 15033189 Review.
Cited by
-
Designed PKC-targeting bryostatin analogs modulate innate immunity and neuroinflammation.Cell Chem Biol. 2021 Apr 15;28(4):537-545.e4. doi: 10.1016/j.chembiol.2020.12.015. Epub 2021 Jan 19. Cell Chem Biol. 2021. PMID: 33472023 Free PMC article.
-
Toll-like receptor 2bright cells identify circulating monocytes in human and non-human primates.Cytometry A. 2017 Apr;91(4):364-371. doi: 10.1002/cyto.a.23098. Epub 2017 Mar 21. Cytometry A. 2017. PMID: 28323396 Free PMC article.
-
Novel Mechanism of Microvesicle Regulation by the Antiviral Protein Tetherin During HIV Infection.J Am Heart Assoc. 2020 Sep;9(17):e015998. doi: 10.1161/JAHA.120.015998. Epub 2020 Aug 21. J Am Heart Assoc. 2020. PMID: 32819189 Free PMC article.
-
Circulating LPS and (1→3)-β-D-Glucan: A Folie à Deux Contributing to HIV-Associated Immune Activation.Front Immunol. 2019 Mar 18;10:465. doi: 10.3389/fimmu.2019.00465. eCollection 2019. Front Immunol. 2019. PMID: 30967860 Free PMC article. Review.
-
Toll-Like Receptor 4 Mediates Methamphetamine-Induced Neuroinflammation through Caspase-11 Signaling Pathway in Astrocytes.Front Mol Neurosci. 2017 Dec 12;10:409. doi: 10.3389/fnmol.2017.00409. eCollection 2017. Front Mol Neurosci. 2017. PMID: 29311802 Free PMC article.
References
-
- Brockman MA, Kwon DS, Tighe DP, Pavlik DF, Rosato PC, Sela J, Porichis F, Le Gall S, Waring MT, Moss K, Jessen H, Pereyra F, Kavanagh DG, Walker BD, Kaufmann DE. 2009. IL-10 is up-regulated in multiple cell types during viremic HIV infection and reversibly inhibits virus-specific T cells. Blood 114:346–356. doi:10.1182/blood-2008-12-191296. - DOI - PMC - PubMed
-
- Chen P, Mayne M, Power C, Nath A. 1997. The Tat protein of HIV-1 induces tumor necrosis factor-alpha production. Implications for HIV-1-associated neurological diseases. J Biol Chem 272:22385–22388. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

