Manipulating TLR Signaling Increases the Anti-tumor T Cell Response Induced by Viral Cancer Therapies
- PMID: 27050526
- PMCID: PMC4830920
- DOI: 10.1016/j.celrep.2016.03.017
Manipulating TLR Signaling Increases the Anti-tumor T Cell Response Induced by Viral Cancer Therapies
Abstract
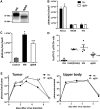
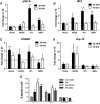
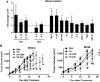
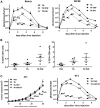
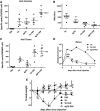
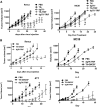
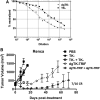
The immune response plays a key role in enhancing the therapeutic activity of oncolytic virotherapies. However, to date, investigators have relied on inherent interactions between the virus and the immune system, often coupled to the expression of a single cytokine transgene. Recently, the importance of TLR activation in mediating adaptive immunity has been demonstrated. We therefore sought to influence the type and level of immune response raised after oncolytic vaccinia therapy through manipulation of TLR signaling. Vaccinia naturally activates TLR2, associated with an antibody response, whereas a CTL response is associated with TLR3-TRIF-signaling pathways. We manipulated TLR signaling by vaccinia through deglycosylation of the viral particle to block TLR2 activation and expression of a TRIF transgene. The resulting vector displayed greatly reduced production of anti-viral neutralizing antibody as well as an increased anti-tumor CTL response. Delivery in both naive and pre-treated mice was enhanced and immunotherapeutic activity dramatically improved.
Copyright © 2016 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Trif is not required for immune complex glomerulonephritis: dying cells activate mesangial cells via Tlr2/Myd88 rather than Tlr3/Trif.Am J Physiol Renal Physiol. 2009 Apr;296(4):F867-74. doi: 10.1152/ajprenal.90213.2008. Epub 2009 Jan 21. Am J Physiol Renal Physiol. 2009. PMID: 19158348
-
Targeting of interferon-beta to produce a specific, multi-mechanistic oncolytic vaccinia virus.PLoS Med. 2007 Dec;4(12):e353. doi: 10.1371/journal.pmed.0040353. PLoS Med. 2007. PMID: 18162040 Free PMC article.
-
Enhancing poxvirus oncolytic effects through increased spread and immune evasion.Cancer Res. 2008 Apr 1;68(7):2071-5. doi: 10.1158/0008-5472.CAN-07-6515. Cancer Res. 2008. PMID: 18381410
-
Cancer relapse under chemotherapy: why TLR2/4 receptor agonists can help.Eur J Pharmacol. 2007 Jun 1;563(1-3):1-17. doi: 10.1016/j.ejphar.2007.02.018. Epub 2007 Feb 17. Eur J Pharmacol. 2007. PMID: 17383632 Review.
-
Pexa-Vec double agent engineered vaccinia: oncolytic and active immunotherapeutic.Curr Opin Virol. 2015 Aug;13:49-54. doi: 10.1016/j.coviro.2015.03.016. Epub 2015 Apr 17. Curr Opin Virol. 2015. PMID: 25900822 Review.
Cited by
-
Oncolytic Viruses and the Immune System: The Dynamic Duo.Mol Ther Methods Clin Dev. 2020 Jan 15;17:349-358. doi: 10.1016/j.omtm.2020.01.001. eCollection 2020 Jun 12. Mol Ther Methods Clin Dev. 2020. PMID: 32071927 Free PMC article. Review.
-
Activation of interferon regulatory factor 3 by replication-competent vaccinia viruses improves antitumor efficacy mediated by T cell responses.Mol Ther Oncolytics. 2021 Jun 4;22:399-409. doi: 10.1016/j.omto.2021.06.001. eCollection 2021 Sep 24. Mol Ther Oncolytics. 2021. PMID: 34553028 Free PMC article.
-
White paper on microbial anti-cancer therapy and prevention.J Immunother Cancer. 2018 Aug 6;6(1):78. doi: 10.1186/s40425-018-0381-3. J Immunother Cancer. 2018. PMID: 30081947 Free PMC article. Review.
-
Targeting Nucleotide Biosynthesis: A Strategy for Improving the Oncolytic Potential of DNA Viruses.Front Oncol. 2017 Sep 26;7:229. doi: 10.3389/fonc.2017.00229. eCollection 2017. Front Oncol. 2017. PMID: 29018771 Free PMC article. Review.
-
Trial Watch: Oncolytic viro-immunotherapy of hematologic and solid tumors.Oncoimmunology. 2018 Aug 27;7(12):e1503032. doi: 10.1080/2162402X.2018.1503032. eCollection 2018. Oncoimmunology. 2018. PMID: 30524901 Free PMC article. Review.
References
-
- Andtbacka RHI, Collichio FA, Amatruda T, Senzer NN, Chesney J, Delman KA, Spitler LE, Puzanov I, Doleman S, Ye Y, et al. OPTiM: A randomized phase III trial of talimogene laherparepvec (T-VEC) versus subcutaneous (SC) granulocyte-macrophage colony-stimulating factor (GM-CSF) for the treatment (tx) of unresected stage IIIB/C and IV melanoma. J. Clin. Oncol. 2013;31:LBA9008.
-
- Chakrabarti S, Sisler JR, Moss B. Compact, synthetic, vaccinia virus early/late promoter for protein expression. Biotechniques. 1997;23:1094–1097. - PubMed
-
- Essajee S, Kaufman HL. Poxvirus vaccines for cancer and HIV therapy. Expert Opin. Biol. Ther. 2004;4:575–588. - PubMed
-
- Ganly I, Kirn D, Eckhardt G, Rodriguez GI, Soutar DS, Otto R, Robertson AG, Park O, Gulley ML, Heise C, et al. A phase I study of Onyx-015, an E1B attenuated adenovirus, administered intratumorally to patients with recurrent head and neck cancer. Clin. Cancer Res. 2000;6:798–806. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

