Stromal fibroblasts support dendritic cells to maintain IL-23/Th17 responses after exposure to ionizing radiation
- PMID: 27049023
- PMCID: PMC4945355
- DOI: 10.1189/jlb.3A1015-474R
Stromal fibroblasts support dendritic cells to maintain IL-23/Th17 responses after exposure to ionizing radiation
Abstract
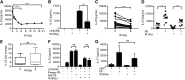
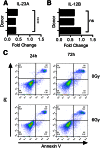
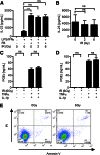
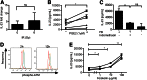
Dendritic cell function is modulated by stromal cells, including fibroblasts. Although poorly understood, the signals delivered through this crosstalk substantially alter dendritic cell biology. This is well illustrated with release of TNF-α/IL-1β from activated dendritic cells, promoting PGE2 secretion from stromal fibroblasts. This instructs dendritic cells to up-regulate IL-23, a key Th17-polarizing cytokine. We previously showed that ionizing radiation inhibited IL-23 production by human dendritic cells in vitro. In the present study, we investigated the hypothesis that dendritic cell-fibroblast crosstalk overcomes the suppressive effect of ionizing radiation to support appropriately polarized Th17 responses. Radiation (1-6 Gy) markedly suppressed IL-23 secretion by activated dendritic cells (P < 0.0001) without adversely impacting their viability and consequently, inhibited the generation of Th17 responses. Cytokine suppression by ionizing radiation was selective, as there was no effect on IL-1β, -6, -10, and -27 or TNF-α and only a modest (11%) decrease in IL-12p70 secretion. Coculture with fibroblasts augmented IL-23 secretion by irradiated dendritic cells and increased Th17 responses. Importantly, in contrast to dendritic cells, irradiated fibroblasts maintained their capacity to respond to TNF-α/IL-1β and produce PGE2, thus providing the key intermediary signals for successful dendritic cell-fibroblasts crosstalk. In summary, stromal fibroblasts support Th17-polarizing cytokine production by dendritic cells that would otherwise be suppressed in an irradiated microenvironment. This has potential ramifications for understanding the immune response to local radiotherapy. These findings underscore the need to account for the impact of microenvironmental factors, including stromal cells, in understanding the control of immunity.
Keywords: cancer; cytokine; immunity.
© The Author(s).
Figures

Similar articles
-
Human fibroblasts support the expansion of IL-17-producing T cells via up-regulation of IL-23 production by dendritic cells.Blood. 2010 Sep 9;116(10):1715-25. doi: 10.1182/blood-2010-01-263509. Epub 2010 Jun 10. Blood. 2010. PMID: 20538798
-
Novel approach for interleukin-23 up-regulation in human dendritic cells and the impact on T helper type 17 generation.Immunology. 2011 Sep;134(1):60-72. doi: 10.1111/j.1365-2567.2011.03467.x. Epub 2011 Jun 30. Immunology. 2011. PMID: 21718315 Free PMC article.
-
The link between IL-23 and Th17 cell-mediated immune pathologies.Semin Immunol. 2007 Dec;19(6):372-6. doi: 10.1016/j.smim.2007.10.012. Epub 2007 Dec 3. Semin Immunol. 2007. PMID: 18319054 Review.
-
Neisseria gonorrhoeae triggers the PGE2/IL-23 pathway and promotes IL-17 production by human memory T cells.Prostaglandins Other Lipid Mediat. 2012 Oct;99(1-2):24-9. doi: 10.1016/j.prostaglandins.2012.04.002. Epub 2012 Apr 20. Prostaglandins Other Lipid Mediat. 2012. PMID: 22542425
-
From interleukin-23 to T-helper 17 cells: human T-helper cell differentiation revisited.Immunol Rev. 2008 Dec;226:132-46. doi: 10.1111/j.1600-065X.2008.00714.x. Immunol Rev. 2008. PMID: 19161421 Free PMC article. Review.
Cited by
-
Multimodal control of dendritic cell functions by nociceptors.Science. 2023 Mar 31;379(6639):eabm5658. doi: 10.1126/science.abm5658. Epub 2023 Mar 31. Science. 2023. PMID: 36996219 Free PMC article.
-
Immune targets in the tumor microenvironment treated by radiotherapy.Theranostics. 2019 Jan 30;9(5):1215-1231. doi: 10.7150/thno.32648. eCollection 2019. Theranostics. 2019. PMID: 30867826 Free PMC article. Review.
-
Stromal C-type lectin receptor COLEC12 integrates H. pylori, PGE2-EP2/4 axis and innate immunity in gastric diseases.Sci Rep. 2018 Feb 28;8(1):3821. doi: 10.1038/s41598-018-20957-2. Sci Rep. 2018. PMID: 29491476 Free PMC article.
-
Fibroblasts in cancer dormancy: foe or friend?Cancer Cell Int. 2021 Mar 26;21(1):184. doi: 10.1186/s12935-021-01883-2. Cancer Cell Int. 2021. PMID: 33771156 Free PMC article. Review.
-
Combination strategies to enhance the potency of monocyte-derived dendritic cell-based cancer vaccines.Immunotherapy. 2016 Oct;8(10):1205-18. doi: 10.2217/imt-2016-0071. Immunotherapy. 2016. PMID: 27605069 Free PMC article. Review.
References
-
- Saalbach A., Klein C., Sleeman J., Sack U., Kauer F., Gebhardt C., Averbeck M., Anderegg U., Simon J. C. (2007) Dermal fibroblasts induce maturation of dendritic cells. J. Immunol. 178, 4966–4974. - PubMed
-
- Saalbach A., Klein C., Schirmer C., Briest W., Anderegg U., Simon J. C. (2010) Dermal fibroblasts promote the migration of dendritic cells. J. Invest. Dermatol. 130, 444–454. - PubMed
-
- Schirmer C., Klein C., von Bergen M., Simon J. C., Saalbach A. (2010) Human fibroblasts support the expansion of IL-17-producing T cells via up-regulation of IL-23 production by dendritic cells. Blood 116, 1715–1725. - PubMed
-
- Comito G., Giannoni E., Segura C. P., Barcellos-de-Souza P., Raspollini M. R., Baroni G., Lanciotti M., Serni S., Chiarugi P. (2014) Cancer-associated fibroblasts and M2-polarized macrophages synergize during prostate carcinoma progression. Oncogene 33, 2423–2431. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

