Comparison of Anti-Oxidant and Anti-Inflammatory Effects between Fresh and Aged Black Garlic Extracts
- PMID: 27043510
- PMCID: PMC6274159
- DOI: 10.3390/molecules21040430
Comparison of Anti-Oxidant and Anti-Inflammatory Effects between Fresh and Aged Black Garlic Extracts
Abstract
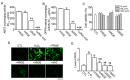
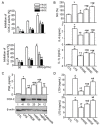
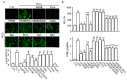
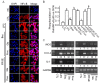
Numerous studies have demonstrated that aged black garlic (ABG) has strong anti-oxidant activity. Little is known however regarding the anti-inflammatory activity of ABG. This study was performed to identify and compare the anti-oxidant and anti-inflammatory effects of ABG extract (ABGE) with those of fresh raw garlic (FRG) extract (FRGE). In addition, we investigated which components are responsible for the observed effects. Hydrogen peroxide (H2O2) and lipopolysaccharide (LPS) were used as a pro-oxidant and pro-inflammatory stressor, respectively. ABGE showed high ABTS and DPPH radical scavenging activities and low ROS generation in RAW264.7 cells compared with FRGE. However, inhibition of cyclooxygenase-2 and 5-lipooxygenase activities by FRGE was stronger than that by ABGE. FRGE reduced PGE₂, NO, IL-6, IL-1β, LTD₄, and LTE₄ production in LPS-activated RAW264.7 cells more than did ABGE. The combination of FRGE and sugar (galactose, glucose, fructose, or sucrose), which is more abundant in ABGE than in FRGE, decreased the anti-inflammatory activity compared with FRGE. FRGE-induced inhibition of NF-κB activation and pro-inflammatory gene expression was blocked by combination with sugars. The lower anti-inflammatory activity in ABGE than FRGE could result from the presence of sugars. Our results suggest that ABGE might be helpful for the treatment of diseases mediated predominantly by ROS.
Keywords: NF-kappa B; anti-inflammatory agents; antioxidants; garlic; sugar.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Short-term heating reduces the anti-inflammatory effects of fresh raw garlic extracts on the LPS-induced production of NO and pro-inflammatory cytokines by downregulating allicin activity in RAW 264.7 macrophages.Food Chem Toxicol. 2013 Aug;58:545-51. doi: 10.1016/j.fct.2013.04.002. Epub 2013 Apr 11. Food Chem Toxicol. 2013. PMID: 23583806
-
The Protective Effects of an Aged Black Garlic Water Extract on the Prostate.Nutrients. 2024 Sep 7;16(17):3025. doi: 10.3390/nu16173025. Nutrients. 2024. PMID: 39275340 Free PMC article.
-
Anti-inflammatory and anti-hyperalgesic effects induced by an aqueous aged black garlic extract in rodent models of ulcerative colitis and colitis-associated visceral pain.Phytother Res. 2024 Aug;38(8):4177-4188. doi: 10.1002/ptr.8270. Epub 2024 Jun 24. Phytother Res. 2024. PMID: 38923108
-
Physicochemical Properties, Biological Activity, Health Benefits, and General Limitations of Aged Black Garlic: A Review.Molecules. 2017 Jun 1;22(6):919. doi: 10.3390/molecules22060919. Molecules. 2017. PMID: 28587168 Free PMC article. Review.
-
Antioxidant health effects of aged garlic extract.J Nutr. 2001 Mar;131(3s):1010S-5S. doi: 10.1093/jn/131.3.1010S. J Nutr. 2001. PMID: 11238807 Review.
Cited by
-
An Innovative Mei-Gin Formula Exerts Anti-Adipogenic and Anti-Obesity Effects in 3T3-L1 Adipocyte and High-Fat Diet-Induced Obese Rats.Foods. 2023 Feb 23;12(5):945. doi: 10.3390/foods12050945. Foods. 2023. PMID: 36900462 Free PMC article.
-
Garlic antagonizes skeletal muscle ischemia reperfusion injury through regulating inflammation, apoptosis and desmin expression in adult male rats.Int J Physiol Pathophysiol Pharmacol. 2019 Aug 15;11(4):126-137. eCollection 2019. Int J Physiol Pathophysiol Pharmacol. 2019. PMID: 31523360 Free PMC article.
-
Two new and effective food-extracted immunomodulatory agents exhibit anti-inflammatory response activity in the hACE2 acute lung injury murine model of COVID-19.Front Immunol. 2024 May 14;15:1374541. doi: 10.3389/fimmu.2024.1374541. eCollection 2024. Front Immunol. 2024. PMID: 38807598 Free PMC article.
-
Effect of Thermal Processes on S-Allyl Cysteine Content in Black Garlic.Foods. 2023 Mar 13;12(6):1227. doi: 10.3390/foods12061227. Foods. 2023. PMID: 36981153 Free PMC article.
-
Alterations in the Physicochemical Properties and Antioxidant Activity during Aging of Stored Raw Garlic.Foods. 2022 May 11;11(10):1390. doi: 10.3390/foods11101390. Foods. 2022. PMID: 35626958 Free PMC article.
References
-
- Freeman B.A., Crapo J.D. Biology of disease: Free radicals and tissue injury. Lab. Investig. 1982;47:412–426. - PubMed
-
- Salzano S., Checconi P., Hanschmann E.M., Lillig C.H., Bowler L.D., Chan P., Vaudry D., Mengozzi M., Coppo L., Sacre S., et al. Linkage of inflammation and oxidative stress via release of glutathionylated peroxiredoxin-2, which acts as a danger signal. Proc. Natl. Acad. Sci. USA. 2014;111:12157–12162. doi: 10.1073/pnas.1401712111. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

