Efficacy of nystatin for the treatment of oral candidiasis: a systematic review and meta-analysis
- PMID: 27042008
- PMCID: PMC4801147
- DOI: 10.2147/DDDT.S100795
Efficacy of nystatin for the treatment of oral candidiasis: a systematic review and meta-analysis
Abstract
Objective: To systematically review and assess the efficacy, different treatment protocols (formulation, dosage, and duration), and safety of nystatin for treating oral candidiasis.
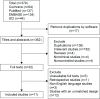
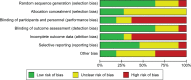
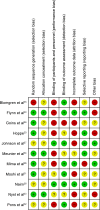
Methods: Four electronic databases were searched for trials published in English till July 1, 2015. Randomized controlled trials comparing nystatin with other antifungal therapies or a placebo were included. Clinical and/or mycological cure was the outcome evaluation. A meta-analysis or descriptive study on the efficacy, treatment protocols, and safety of nystatin was conducted.
Results: The meta-analysis showed that nystatin pastille was significantly superior to placebo in treating denture stomatitis. Nystatin suspension was not superior to fluconazole in treating oral candidiasis in infants, children, or HIV/AIDS patients. The descriptive investigations showed that administration of nystatin suspension and pastilles in combination for 2 weeks might achieve a higher clinical and mycological cure rate, and using the nystatin pastilles alone might have a higher mycological cure rate, when compared with using nystatin suspensions alone. Nystatin pastilles at a dose of 400,000 IU resulted in a significantly higher mycological cure rate than that administrated at a dose of 200,000 IU. Furthermore, treatment with nystatin pastilles for 4 weeks seemed to have better clinical efficacy than treatment for 2 weeks. Descriptive safety assessment showed that poor taste and gastrointestinal adverse reaction are the most common adverse effects of nystatin.
Conclusion: Nystatin pastille was significantly superior to placebo in treating denture stomatitis, while nystatin suspension was not superior to fluconazole in treating oral candidiasis in infants, children, or HIV/AIDS patients. Indirect evidence from a descriptive study demonstrated that administration of nystatin pastille alone or pastille and suspension in combination is more effective than that of suspension alone; prolonged treatment duration for up to 4 weeks can increase the efficacy of nystatin. More well designed and high quality randomized control studies are needed to confirm these findings.
Keywords: dosage forms; meta-analysis; nystatin; oral candidiasis; safety; systematic review; treatment duration.
Figures

Similar articles
-
Effects of Streptococcus salivarius K12 with nystatin on oral candidiasis-RCT.Oral Dis. 2019 Sep;25(6):1573-1580. doi: 10.1111/odi.13142. Epub 2019 Jul 26. Oral Dis. 2019. PMID: 31177581 Clinical Trial.
-
Clinical evaluation of a nystatin pastille for treatment of denture-related oral candidiasis.J Prosthet Dent. 1989 Jun;61(6):699-703. doi: 10.1016/s0022-3913(89)80045-9. J Prosthet Dent. 1989. PMID: 2657027 Clinical Trial.
-
Efficacy of photodynamic therapy in the treatment of oral candidiasis: a systematic review and meta-analysis.BMC Oral Health. 2023 Oct 26;23(1):802. doi: 10.1186/s12903-023-03484-z. BMC Oral Health. 2023. PMID: 37884914 Free PMC article.
-
Nystatin Effectiveness in Oral Candidiasis Treatment: A Systematic Review & Meta-Analysis of Clinical Trials.Life (Basel). 2022 Oct 22;12(11):1677. doi: 10.3390/life12111677. Life (Basel). 2022. PMID: 36362833 Free PMC article. Review.
-
Comparative efficacy of antifungal drugs for the treatment of oral candidiasis in HIV-positive patients: A Bayesian network meta-analysis.Med Clin (Barc). 2024 Aug 29:S0025-7753(24)00393-2. doi: 10.1016/j.medcli.2024.05.018. Online ahead of print. Med Clin (Barc). 2024. PMID: 39214731 Review. English, Spanish.
Cited by
-
Investigating in-vitro functionality and in-vivo taste assessment of eco-friendly Tadalafil Pastilles.Heliyon. 2024 Apr 16;10(8):e29543. doi: 10.1016/j.heliyon.2024.e29543. eCollection 2024 Apr 30. Heliyon. 2024. PMID: 38660288 Free PMC article.
-
In Vitro Assessment of Antifungal and Antibiofilm Efficacy of Commercial Mouthwashes against Candida albicans.Antibiotics (Basel). 2024 Jan 25;13(2):117. doi: 10.3390/antibiotics13020117. Antibiotics (Basel). 2024. PMID: 38391503 Free PMC article.
-
Multilocus sequence typing of Candida albicans oral isolates reveals high genetic relatedness of mother-child dyads in early life.PLoS One. 2024 Jan 17;19(1):e0290938. doi: 10.1371/journal.pone.0290938. eCollection 2024. PLoS One. 2024. PMID: 38232064 Free PMC article.
-
[Clinical features of oral management to oral complications of Sjögren's syndrome].Beijing Da Xue Xue Bao Yi Xue Ban. 2023 Oct 18;55(5):929-933. doi: 10.19723/j.issn.1671-167X.2023.05.023. Beijing Da Xue Xue Bao Yi Xue Ban. 2023. PMID: 37807750 Free PMC article. Chinese.
-
Antimicrobial Activity of Selenium Nanoparticles (SeNPs) against Potentially Pathogenic Oral Microorganisms: A Scoping Review.Pharmaceutics. 2023 Aug 31;15(9):2253. doi: 10.3390/pharmaceutics15092253. Pharmaceutics. 2023. PMID: 37765222 Free PMC article. Review.
References
-
- Melkoumov A, Goupil M, Louhichi F, Raymond M, de Repentigny L, Leclair G. Nystatin nanosizing enhances in vitro and in vivo antifungal activity against Candida albicans. J Antimicrob Chemother. 2013;68(9):2099–2105. - PubMed
-
- Epstein JB, Polsky B. Oropharyngeal candidiasis: a review of its clinical spectrum and current therapies. Clin Ther. 1998;20(1):40–57. - PubMed
-
- Samaranayake LP, Keung Leung W, Jin L. Oral mucosal fungal infections. Periodontol 2000. 2009;49:39–59. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

