Extracellular vesicles and intercellular communication within the nervous system
- PMID: 27035811
- PMCID: PMC4811121
- DOI: 10.1172/JCI81134
Extracellular vesicles and intercellular communication within the nervous system
Abstract
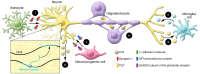
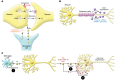
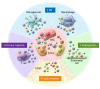
Extracellular vesicles (EVs, including exosomes) are implicated in many aspects of nervous system development and function, including regulation of synaptic communication, synaptic strength, and nerve regeneration. They mediate the transfer of packets of information in the form of nonsecreted proteins and DNA/RNA protected within a membrane compartment. EVs are essential for the packaging and transport of many cell-fate proteins during development as well as many neurotoxic misfolded proteins during pathogenesis. This form of communication provides another dimension of cellular crosstalk, with the ability to assemble a "kit" of directional instructions made up of different molecular entities and address it to specific recipient cells. This multidimensional form of communication has special significance in the nervous system. How EVs help to orchestrate the wiring of the brain while allowing for plasticity associated with learning and memory and contribute to regeneration and degeneration are all under investigation. Because they carry specific disease-related RNAs and proteins, practical applications of EVs include potential uses as biomarkers and therapeutics. This Review describes our current understanding of EVs and serves as a springboard for future advances, which may reveal new important mechanisms by which EVs in coordinate brain and body function and dysfunction.
Figures
Similar articles
-
Extracellular vesicles: masters of intercellular communication and potential clinical interventions.J Clin Invest. 2016 Apr 1;126(4):1139-43. doi: 10.1172/JCI87316. Epub 2016 Apr 1. J Clin Invest. 2016. PMID: 27035805 Free PMC article. Review.
-
Transcriptome of Extracellular Vesicles: State-of-the-Art.Front Immunol. 2019 Feb 28;10:202. doi: 10.3389/fimmu.2019.00202. eCollection 2019. Front Immunol. 2019. PMID: 30873152 Free PMC article. Review.
-
Versatile roles of extracellular vesicles in cancer.J Clin Invest. 2016 Apr 1;126(4):1163-72. doi: 10.1172/JCI81130. Epub 2016 Mar 14. J Clin Invest. 2016. PMID: 26974161 Free PMC article. Review.
-
Emerging role of extracellular vesicles as a senescence-associated secretory phenotype: Insights into the pathophysiology of lung diseases.Mol Aspects Med. 2018 Apr;60:92-103. doi: 10.1016/j.mam.2017.11.005. Epub 2017 Nov 20. Mol Aspects Med. 2018. PMID: 29146100 Review.
-
Cardioprotective role of extracellular vesicles: A highlight on exosome beneficial effects in cardiovascular diseases.J Cell Physiol. 2019 Dec;234(12):21732-21745. doi: 10.1002/jcp.28894. Epub 2019 May 29. J Cell Physiol. 2019. PMID: 31140622 Review.
Cited by
-
Inter and Intracellular mitochondrial trafficking in health and disease.Ageing Res Rev. 2020 Sep;62:101128. doi: 10.1016/j.arr.2020.101128. Epub 2020 Jul 23. Ageing Res Rev. 2020. PMID: 32712108 Free PMC article. Review.
-
Extracellular Vesicle-Mediated Communication between the Glioblastoma and Its Microenvironment.Cells. 2019 Dec 30;9(1):96. doi: 10.3390/cells9010096. Cells. 2019. PMID: 31906023 Free PMC article. Review.
-
Application of Mesenchymal Stem Cells in Targeted Delivery to the Brain: Potential and Challenges of the Extracellular Vesicle-Based Approach for Brain Tumor Treatment.Int J Mol Sci. 2021 Oct 17;22(20):11187. doi: 10.3390/ijms222011187. Int J Mol Sci. 2021. PMID: 34681842 Free PMC article. Review.
-
Proteomic and lipidomic analysis of exosomes derived from ovarian cancer cells and ovarian surface epithelial cells.J Ovarian Res. 2020 Jan 22;13(1):9. doi: 10.1186/s13048-020-0609-y. J Ovarian Res. 2020. PMID: 31969186 Free PMC article.
-
Potential Transfer of Polyglutamine and CAG-Repeat RNA in Extracellular Vesicles in Huntington's Disease: Background and Evaluation in Cell Culture.Cell Mol Neurobiol. 2016 Apr;36(3):459-70. doi: 10.1007/s10571-016-0350-7. Epub 2016 Mar 7. Cell Mol Neurobiol. 2016. PMID: 26951563 Free PMC article.
References
-
- Rilla K, Siiskonen H, Tammi M, Tammi R. Hyaluronan-coated extracellular vesicles — a novel link between hyaluronan and cancer. Adv Cancer Res. 2014;123:121–148. - PubMed
-
- Marzesco AM, et al. Release of extracellular membrane particles carrying the stem cell marker prominin-1 (CD133) from neural progenitors and other epithelial cells. J Cell Sci. 2005;118(pt 13):2849–2858. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

