Double Roles of Macrophages in Human Neuroimmune Diseases and Their Animal Models
- PMID: 27034594
- PMCID: PMC4808549
- DOI: 10.1155/2016/8489251
Double Roles of Macrophages in Human Neuroimmune Diseases and Their Animal Models
Abstract
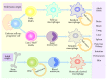
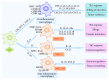
Macrophages are important immune cells of the innate immune system that are involved in organ-specific homeostasis and contribute to both pathology and resolution of diseases including infections, cancer, obesity, atherosclerosis, and autoimmune disorders. Multiple lines of evidence point to macrophages as a remarkably heterogeneous cell type. Different phenotypes of macrophages exert either proinflammatory or anti-inflammatory roles depending on the cytokines and other mediators that they are exposed to in the local microenvironment. Proinflammatory macrophages secrete detrimental molecules to induce disease development, while anti-inflammatory macrophages produce beneficial mediators to promote disease recovery. The conversion of the phenotypes of macrophages can regulate the initiation, development, and recovery of autoimmune diseases. Human neuroimmune diseases majorly include multiple sclerosis (MS), neuromyelitis optica (NMO), myasthenia gravis (MG), and Guillain-Barré syndrome (GBS) and macrophages contribute to the pathogenesis of these neuroimmune diseases. In this review, we summarize the double roles of macrophage in neuroimmune diseases and their animal models to further explore the mechanisms of macrophages involved in the pathogenesis of these disorders, which may provide a potential therapeutic approach for these disorders in the future.
Figures
Similar articles
-
Beneficial or Harmful Role of Macrophages in Guillain-Barré Syndrome and Experimental Autoimmune Neuritis.Mediators Inflamm. 2018 Apr 26;2018:4286364. doi: 10.1155/2018/4286364. eCollection 2018. Mediators Inflamm. 2018. PMID: 29853789 Free PMC article. Review.
-
[Neuroimmunological diseases: update].Nihon Rinsho. 2013 May;71(5):771-7. Nihon Rinsho. 2013. PMID: 23777080 Review. Japanese.
-
NKT and NKT-like Cells in Autoimmune Neuroinflammatory Diseases-Multiple Sclerosis, Myasthenia Gravis and Guillain-Barre Syndrome.Int J Mol Sci. 2021 Sep 1;22(17):9520. doi: 10.3390/ijms22179520. Int J Mol Sci. 2021. PMID: 34502425 Free PMC article. Review.
-
Complement biomarkers reflect the pathological status of neuromyelitis optica spectrum disorders.Front Immunol. 2023 Mar 3;14:1090548. doi: 10.3389/fimmu.2023.1090548. eCollection 2023. Front Immunol. 2023. PMID: 36936980 Free PMC article.
-
Friendly fire on neurons: antibody-mediated diseases of the nervous system.Ceylon Med J. 2015 Dec;60(4):121-5. doi: 10.4038/cmj.v60i4.8218. Ceylon Med J. 2015. PMID: 26778390 No abstract available.
Cited by
-
Myeloid cell plasticity in the evolution of central nervous system autoimmunity.Ann Neurol. 2018 Jan;83(1):131-141. doi: 10.1002/ana.25128. Epub 2018 Jan 14. Ann Neurol. 2018. PMID: 29283442 Free PMC article.
-
TAMeless traitors: macrophages in cancer progression and metastasis.Br J Cancer. 2017 Nov 21;117(11):1583-1591. doi: 10.1038/bjc.2017.356. Epub 2017 Oct 24. Br J Cancer. 2017. PMID: 29065107 Free PMC article. Review.
-
Overview of diet and autoimmune demyelinating optic neuritis: a narrative review.Immunometabolism (Cobham). 2023 Apr 27;5(2):e00022. doi: 10.1097/IN9.0000000000000022. eCollection 2023 Apr. Immunometabolism (Cobham). 2023. PMID: 37128292 Free PMC article. Review.
-
A Mathematical Model of Breast Tumor Progression Based on Immune Infiltration.J Pers Med. 2021 Oct 15;11(10):1031. doi: 10.3390/jpm11101031. J Pers Med. 2021. PMID: 34683171 Free PMC article.
-
Serum irisin levels in patients with myasthenia gravis.Neurol Sci. 2022 Apr;43(4):2785-2790. doi: 10.1007/s10072-021-05652-x. Epub 2021 Oct 28. Neurol Sci. 2022. PMID: 34709479
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

