Local proliferation initiates macrophage accumulation in adipose tissue during obesity
- PMID: 27031964
- PMCID: PMC4823955
- DOI: 10.1038/cddis.2016.54
Local proliferation initiates macrophage accumulation in adipose tissue during obesity
Abstract
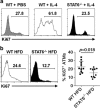
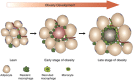
Obesity-associated chronic inflammation is characterized by an accumulation of adipose tissue macrophages (ATMs). It is generally believed that those macrophages are derived from peripheral blood monocytes. However, recent studies suggest that local proliferation of macrophages is responsible for ATM accumulation. In the present study, we revealed that both migration and proliferation contribute to ATM accumulation during obesity development. We show that there is a significant increase in ATMs at the early stage of obesity, which is largely due to an enhanced in situ macrophage proliferation. This result was obtained by employing fat-shielded irradiation and bone marrow reconstitution. Additionally, the production of CCL2, a pivotal chemoattractant of monocytes, was not found to be increased at this stage, corroborating with a critical role of proliferation. Nonetheless, as obesity proceeds, the role of monocyte migration into adipose tissue becomes more significant and those new immigrants further proliferate locally. These proliferating ATMs mainly reside in crown-like structures formed by macrophages surrounding dead adipocytes. We further showed that IL-4/STAT6 is a driving force for ATM proliferation. Therefore, we demonstrated that local proliferation of resident macrophages contributes to ATM accumulation during obesity development and has a key role in obesity-associated inflammation.
Figures




Similar articles
-
CD11b regulates obesity-induced insulin resistance via limiting alternative activation and proliferation of adipose tissue macrophages.Proc Natl Acad Sci U S A. 2015 Dec 29;112(52):E7239-48. doi: 10.1073/pnas.1500396113. Epub 2015 Dec 15. Proc Natl Acad Sci U S A. 2015. PMID: 26669445 Free PMC article.
-
Frontline Science: Rapid adipose tissue expansion triggers unique proliferation and lipid accumulation profiles in adipose tissue macrophages.J Leukoc Biol. 2018 Apr;103(4):615-628. doi: 10.1002/JLB.3HI1017-422R. Epub 2018 Mar 1. J Leukoc Biol. 2018. PMID: 29493813 Free PMC article.
-
IL-6 Regulates M2 Polarization and Local Proliferation of Adipose Tissue Macrophages in Obesity.J Immunol. 2017 Apr 1;198(7):2927-2934. doi: 10.4049/jimmunol.1600476. Epub 2017 Feb 13. J Immunol. 2017. PMID: 28193830
-
Insights into the role of macrophage migration inhibitory factor in obesity and insulin resistance.Proc Nutr Soc. 2012 Nov;71(4):622-33. doi: 10.1017/S0029665112000730. Epub 2012 Aug 22. Proc Nutr Soc. 2012. PMID: 22914223 Review.
-
The "Big Bang" in obese fat: Events initiating obesity-induced adipose tissue inflammation.Eur J Immunol. 2015 Sep;45(9):2446-56. doi: 10.1002/eji.201545502. Epub 2015 Aug 19. Eur J Immunol. 2015. PMID: 26220361 Review.
Cited by
-
Tumour biology of obesity-related cancers: understanding the molecular concept for better diagnosis and treatment.Tumour Biol. 2016 Nov;37(11):14363-14380. doi: 10.1007/s13277-016-5357-7. Epub 2016 Sep 14. Tumour Biol. 2016. PMID: 27623943 Review.
-
Macrophages inhibit adipogenic differentiation of adipose tissue derived mesenchymal stem/stromal cells by producing pro-inflammatory cytokines.Cell Biosci. 2020 Jul 20;10:88. doi: 10.1186/s13578-020-00450-y. eCollection 2020. Cell Biosci. 2020. PMID: 32699606 Free PMC article.
-
Innate Immune Cells in the Adipose Tissue in Health and Metabolic Disease.J Innate Immun. 2022;14(1):4-30. doi: 10.1159/000515117. Epub 2021 Apr 13. J Innate Immun. 2022. PMID: 33849008 Free PMC article. Review.
-
The Influence of Metabolism on Immune Response: A Journey to Understand Immunometabolism in the Context of Viral Infection.Viruses. 2023 Dec 9;15(12):2399. doi: 10.3390/v15122399. Viruses. 2023. PMID: 38140640 Free PMC article. Review.
-
Cross-Talk between Inflammatory Mediators and the Epithelial Mesenchymal Transition Process in the Development of Thyroid Carcinoma.Int J Mol Sci. 2019 May 18;20(10):2466. doi: 10.3390/ijms20102466. Int J Mol Sci. 2019. PMID: 31109060 Free PMC article. Review.
References
-
- Johnson AMF, Olefsky JM. The origins and drivers of insulin resistance. Cell 2013; 152: 673–684. - PubMed
-
- Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 1993; 259: 87–91. - PubMed
-
- Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature 1997; 389: 610–614. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

