Evolution of Antibody-Drug Conjugate Tumor Disposition Model to Predict Preclinical Tumor Pharmacokinetics of Trastuzumab-Emtansine (T-DM1)
- PMID: 27029797
- PMCID: PMC5234806
- DOI: 10.1208/s12248-016-9904-3
Evolution of Antibody-Drug Conjugate Tumor Disposition Model to Predict Preclinical Tumor Pharmacokinetics of Trastuzumab-Emtansine (T-DM1)
Abstract
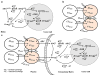
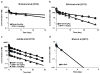
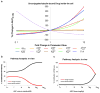
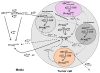
A mathematical model capable of accurately characterizing intracellular disposition of ADCs is essential for a priori predicting unconjugated drug concentrations inside the tumor. Towards this goal, the objectives of this manuscript were to: (1) evolve previously published cellular disposition model of ADC with more intracellular details to characterize the disposition of T-DM1 in different HER2 expressing cell lines, (2) integrate the improved cellular model with the ADC tumor disposition model to a priori predict DM1 concentrations in a preclinical tumor model, and (3) identify prominent pathways and sensitive parameters associated with intracellular activation of ADCs. The cellular disposition model was augmented by incorporating intracellular ADC degradation and passive diffusion of unconjugated drug across tumor cells. Different biomeasures and chemomeasures for T-DM1, quantified in the companion manuscript, were incorporated into the modified model of ADC to characterize in vitro pharmacokinetics of T-DM1 in three HER2+ cell lines. When the cellular model was integrated with the tumor disposition model, the model was able to a priori predict tumor DM1 concentrations in xenograft mice. Pathway analysis suggested different contribution of antigen-mediated and passive diffusion pathways for intracellular unconjugated drug exposure between in vitro and in vivo systems. Global and local sensitivity analyses revealed that non-specific deconjugation and passive diffusion of the drug across tumor cell membrane are key parameters for drug exposure inside a cell. Finally, a systems pharmacokinetic model for intracellular processing of ADCs has been proposed to highlight our current understanding about the determinants of ADC activation inside a cell.
Keywords: T-DM1; antibody-drug conjugate; cellular pharmacokinetics; global sensitivity analysis; mechanistic model; model-based drug development; tumor disposition; tumor pharmacokinetics.
Figures



Comment on
-
Antibody-maytansinoid conjugates are activated in targeted cancer cells by lysosomal degradation and linker-dependent intracellular processing.Cancer Res. 2006 Apr 15;66(8):4426-33. doi: 10.1158/0008-5472.CAN-05-4489. Cancer Res. 2006. PMID: 16618769
-
Modeling the efficacy of trastuzumab-DM1, an antibody drug conjugate, in mice.J Pharmacokinet Pharmacodyn. 2010 Jun;37(3):221-42. doi: 10.1007/s10928-010-9156-2. Epub 2010 Apr 28. J Pharmacokinet Pharmacodyn. 2010. PMID: 20424896
-
Predicting the F(ab)-mediated effect of monoclonal antibodies in vivo by combining cell-level kinetic and pharmacokinetic modelling.J Pharmacokinet Pharmacodyn. 2012 Apr;39(2):125-39. doi: 10.1007/s10928-012-9243-7. Epub 2012 Mar 8. J Pharmacokinet Pharmacodyn. 2012. PMID: 22399130 Free PMC article.
-
The effect of different linkers on target cell catabolism and pharmacokinetics/pharmacodynamics of trastuzumab maytansinoid conjugates.Mol Cancer Ther. 2012 May;11(5):1133-42. doi: 10.1158/1535-7163.MCT-11-0727. Epub 2012 Mar 9. Mol Cancer Ther. 2012. PMID: 22408268
-
Catabolic fate and pharmacokinetic characterization of trastuzumab emtansine (T-DM1): an emphasis on preclinical and clinical catabolism.Curr Drug Metab. 2012 Sep 1;13(7):901-10. doi: 10.2174/138920012802138598. Curr Drug Metab. 2012. PMID: 22475269
-
Mechanistic pharmacokinetic/pharmacodynamic modeling of in vivo tumor uptake, catabolism, and tumor response of trastuzumab maytansinoid conjugates.Cancer Chemother Pharmacol. 2014 Nov;74(5):969-80. doi: 10.1007/s00280-014-2561-2. Epub 2014 Sep 4. Cancer Chemother Pharmacol. 2014. PMID: 25186956
Similar articles
-
An integrated multiple-analyte pharmacokinetic model to characterize trastuzumab emtansine (T-DM1) clearance pathways and to evaluate reduced pharmacokinetic sampling in patients with HER2-positive metastatic breast cancer.Clin Pharmacokinet. 2013 Aug;52(8):657-72. doi: 10.1007/s40262-013-0060-y. Clin Pharmacokinet. 2013. PMID: 23553425
-
A mechanistic pharmacokinetic model elucidating the disposition of trastuzumab emtansine (T-DM1), an antibody-drug conjugate (ADC) for treatment of metastatic breast cancer.AAPS J. 2014 Sep;16(5):994-1008. doi: 10.1208/s12248-014-9618-3. Epub 2014 Jun 11. AAPS J. 2014. PMID: 24917179 Free PMC article.
-
The effect of different linkers on target cell catabolism and pharmacokinetics/pharmacodynamics of trastuzumab maytansinoid conjugates.Mol Cancer Ther. 2012 May;11(5):1133-42. doi: 10.1158/1535-7163.MCT-11-0727. Epub 2012 Mar 9. Mol Cancer Ther. 2012. PMID: 22408268
-
Ado-trastuzumab emtansine: a HER2-positive targeted antibody-drug conjugate.Ann Pharmacother. 2014 Nov;48(11):1484-93. doi: 10.1177/1060028014545354. Epub 2014 Jul 31. Ann Pharmacother. 2014. PMID: 25082874 Review.
-
Trastuzumab emtansine: a unique antibody-drug conjugate in development for human epidermal growth factor receptor 2-positive cancer.Clin Cancer Res. 2011 Oct 15;17(20):6437-47. doi: 10.1158/1078-0432.CCR-11-0762. Clin Cancer Res. 2011. PMID: 22003071 Review.
Cited by
-
Pharmacokinetics and Pharmacodynamics of TAK-164 Antibody Drug Conjugate Coadministered with Unconjugated Antibody.AAPS J. 2022 Oct 7;24(6):107. doi: 10.1208/s12248-022-00756-4. AAPS J. 2022. PMID: 36207468 Free PMC article.
-
Clinical validation of translational antibody PBPK model using tissue distribution data generated with 89Zr-immuno-PET imaging.J Pharmacokinet Pharmacodyn. 2023 Oct;50(5):377-394. doi: 10.1007/s10928-023-09869-5. Epub 2023 Jun 29. J Pharmacokinet Pharmacodyn. 2023. PMID: 37382712
-
Antibody-Drug Conjugates: Pharmacokinetic/Pharmacodynamic Modeling, Preclinical Characterization, Clinical Studies, and Lessons Learned.Clin Pharmacokinet. 2018 Jun;57(6):687-703. doi: 10.1007/s40262-017-0619-0. Clin Pharmacokinet. 2018. PMID: 29188435 Free PMC article. Review.
-
Quantitative evaluation of trastuzumab deruxtecan pharmacokinetics and pharmacodynamics in mouse models of varying degrees of HER2 expression.CPT Pharmacometrics Syst Pharmacol. 2024 Jun;13(6):994-1005. doi: 10.1002/psp4.13133. Epub 2024 Mar 26. CPT Pharmacometrics Syst Pharmacol. 2024. PMID: 38532525 Free PMC article.
-
Quantitative characterization of in vitro bystander effect of antibody-drug conjugates.J Pharmacokinet Pharmacodyn. 2016 Dec;43(6):567-582. doi: 10.1007/s10928-016-9495-8. Epub 2016 Sep 26. J Pharmacokinet Pharmacodyn. 2016. PMID: 27670282 Free PMC article.
References
-
- Sohayla Rostami IQ, Sikorski Robert. The clinical landscape of antibody-drug conjugates. 2014 Available from: http://adcreview.com/articles/doi-10-14229jadc-2014-8-1-001/
-
- Singh AP, Shin YG, Shah DK. Application of pharmacokinetic-pharmacodynamic modeling and simulation for antibody-drug conjugate development. Pharm Res. 2015 - PubMed
-
- Lin K, Tibbitts J. Pharmacokinetic considerations for antibody drug conjugates. Pharm Res. 2012;29(9):2354–66. - PubMed
-
- Shah DK, Haddish-Berhane N, Betts A. Bench to bedside translation of antibody drug conjugates using a multiscale mechanistic PK/PD model: a case study with brentuximab-vedotin. J Pharmacokinet Pharmacodyn. 2012;39(6):643–59. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

