Radial glia inhibit peripheral glial infiltration into the spinal cord at motor exit point transition zones
- PMID: 27029762
- PMCID: PMC4910827
- DOI: 10.1002/glia.22987
Radial glia inhibit peripheral glial infiltration into the spinal cord at motor exit point transition zones
Abstract
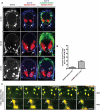
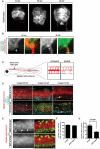
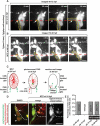
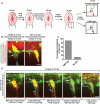
In the mature vertebrate nervous system, central and peripheral nervous system (CNS and PNS, respectively) GLIA myelinate distinct motor axon domains at the motor exit point transition zone (MEP TZ). How these cells preferentially associate with and myelinate discrete, non-overlapping CNS versus PNS axonal segments, is unknown. Using in vivo imaging and genetic cell ablation in zebrafish, we demonstrate that radial glia restrict migration of PNS glia into the spinal cord during development. Prior to development of radial glial endfeet, peripheral cells freely migrate back and forth across the MEP TZ. However, upon maturation, peripherally located cells never enter the CNS. When we ablate radial glia, peripheral glia ectopically migrate into the spinal cord during developmental stages when they would normally be restricted. These findings demonstrate that radial glia contribute to both CNS and PNS development and control the unidirectional movement of glial cell types across the MEP TZ early in development. GLIA 2016. GLIA 2016;64:1138-1153.
Keywords: CNS/PNS transition zone; motor exit point glia; neural crest cells; radial glia; zebrafish.
© 2016 Wiley Periodicals, Inc.
Figures




Similar articles
-
Contact-mediated inhibition between oligodendrocyte progenitor cells and motor exit point glia establishes the spinal cord transition zone.PLoS Biol. 2014 Sep 30;12(9):e1001961. doi: 10.1371/journal.pbio.1001961. eCollection 2014 Sep. PLoS Biol. 2014. PMID: 25268888 Free PMC article.
-
Spinal cord precursors utilize neural crest cell mechanisms to generate hybrid peripheral myelinating glia.Elife. 2021 Feb 8;10:e64267. doi: 10.7554/eLife.64267. Elife. 2021. PMID: 33554855 Free PMC article.
-
Gfap-positive radial glial cells are an essential progenitor population for later-born neurons and glia in the zebrafish spinal cord.Glia. 2016 Jul;64(7):1170-89. doi: 10.1002/glia.22990. Epub 2016 Apr 21. Glia. 2016. PMID: 27100776 Free PMC article.
-
Motor Exit Point (MEP) Glia: Novel Myelinating Glia That Bridge CNS and PNS Myelin.Front Cell Neurosci. 2018 Oct 2;12:333. doi: 10.3389/fncel.2018.00333. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30356886 Free PMC article. Review.
-
Glial plasticity at nervous system transition zones.Biol Open. 2023 Oct 15;12(10):bio060037. doi: 10.1242/bio.060037. Epub 2023 Oct 3. Biol Open. 2023. PMID: 37787575 Free PMC article. Review.
Cited by
-
Single-cell Photoconversion in Living Intact Zebrafish.J Vis Exp. 2018 Mar 19;(133):57024. doi: 10.3791/57024. J Vis Exp. 2018. PMID: 29608164 Free PMC article.
-
Functional Regeneration of the Sensory Root via Axonal Invasion.Cell Rep. 2020 Jan 7;30(1):9-17.e3. doi: 10.1016/j.celrep.2019.12.008. Cell Rep. 2020. PMID: 31914401 Free PMC article.
-
Engulfed by Glia: Glial Pruning in Development, Function, and Injury across Species.J Neurosci. 2021 Feb 3;41(5):823-833. doi: 10.1523/JNEUROSCI.1660-20.2020. Epub 2021 Jan 19. J Neurosci. 2021. PMID: 33468571 Free PMC article. Review.
-
Microglia cannibalism and efferocytosis leads to shorter lifespans of developmental microglia.bioRxiv [Preprint]. 2024 Aug 29:2023.03.15.532426. doi: 10.1101/2023.03.15.532426. bioRxiv. 2024. Update in: PLoS Biol. 2024 Oct 30;22(10):e3002819. doi: 10.1371/journal.pbio.3002819 PMID: 36993267 Free PMC article. Updated. Preprint.
-
More Than Mortar: Glia as Architects of Nervous System Development and Disease.Front Cell Dev Biol. 2020 Dec 14;8:611269. doi: 10.3389/fcell.2020.611269. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33381506 Free PMC article. Review.
References
-
- Bernardos RL, Raymond PA. GFAP transgenic zebrafish. Gene Expression Patterns. 2006;6:1007–1013. - PubMed
-
- Blakemore WF. Invasion of Schwann cells into the spinal cord of the rat following local injections of lysolecithin. Neuropathol Appl Neurobiol. 1976;2:21–39.
-
- Blakemore WF, Patterson RC. Observations on the interactions of Schwann cells and astrocytes following X-irradiation of neonatal rat spinal cord. J Neurocytol. 1975;4:573–585. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

