Natural killer cells regulate Th1/Treg and Th17/Treg balance in chlamydial lung infection
- PMID: 27028780
- PMCID: PMC4929289
- DOI: 10.1111/jcmm.12821
Natural killer cells regulate Th1/Treg and Th17/Treg balance in chlamydial lung infection
Abstract
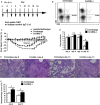
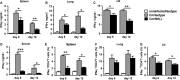
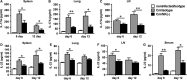
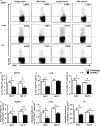
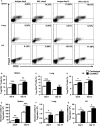
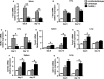
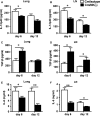
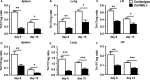
Natural killer (NK) cell is an important component in innate immunity, playing a critical role in bridging innate and adaptive immunity by modulating the function of other immune cells including T cells. In this study, we focused on the role of NK cells in regulating Th1/Treg and Th17/Treg balance during chlamydial lung infection. We found that NK cell-depleted mice showed decreased Th1 and Th17 cells, which was correlated with reduced interferon-γ, interleukin (IL)-12, IL-17 and IL-22 production as well as T-bet and receptor-related orphan receptor gamma t expression compared with mice treated with the isotype control antibody. In contrast, NK cell depletion significantly increased Treg in cell number and related transcription factor (Foxp3) expression. The opposite trends of changes of Th1/Th17 and Treg led to significant reduction in the Th1/Treg and Th17/Treg ratios. The data implicate that NK cells play an important role in host defence against chlamydial lung infection, mainly through maintaining Th1/Treg and Th17/Treg balance.
Keywords: Chlamydia; Th1/Treg; Th17/Treg; immunoregulation; natural killer cells.
© 2016 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Figures
Similar articles
-
NK cells modulate the lung dendritic cell-mediated Th1/Th17 immunity during intracellular bacterial infection.Eur J Immunol. 2015 Oct;45(10):2810-20. doi: 10.1002/eji.201445390. Epub 2015 Aug 24. Eur J Immunol. 2015. PMID: 26222048
-
Scutellaria barbata D. Don extract inhibits the tumor growth through down-regulating of Treg cells and manipulating Th1/Th17 immune response in hepatoma H22-bearing mice.BMC Complement Altern Med. 2017 Jan 13;17(1):41. doi: 10.1186/s12906-016-1551-9. BMC Complement Altern Med. 2017. PMID: 28086772 Free PMC article.
-
[Relationship between CD4 + CD25 + Foxp3 + regulatory T cells and Th17 responses in Chlamydia muridarum pulmonary infection].Wei Sheng Wu Xue Bao. 2013 Jan 4;53(1):74-81. Wei Sheng Wu Xue Bao. 2013. PMID: 23614243 Chinese.
-
Stability of Regulatory T Cells Undermined or Endorsed by Different Type-1 Cytokines.Adv Exp Med Biol. 2015;850:17-30. doi: 10.1007/978-3-319-15774-0_2. Adv Exp Med Biol. 2015. PMID: 26324343 Review.
-
Emerging Concepts of Adaptive Immunity in Leprosy.Front Immunol. 2018 Apr 9;9:604. doi: 10.3389/fimmu.2018.00604. eCollection 2018. Front Immunol. 2018. PMID: 29686668 Free PMC article. Review.
Cited by
-
NK Cell-Mediated Processing Of Chlamydia psittaci Drives Potent Anti-Bacterial Th1 Immunity.Sci Rep. 2019 Mar 18;9(1):4799. doi: 10.1038/s41598-019-41264-4. Sci Rep. 2019. PMID: 30886314 Free PMC article.
-
Spontaneous colitis in IL-10-deficient mice was ameliorated via inhibiting glutaminase1.J Cell Mol Med. 2019 Aug;23(8):5632-5641. doi: 10.1111/jcmm.14471. Epub 2019 Jun 18. J Cell Mol Med. 2019. PMID: 31211512 Free PMC article.
-
Reference Values for a Panel of Cytokinergic and Regulatory Lymphocyte Subpopulations.Immune Netw. 2016 Dec;16(6):344-357. doi: 10.4110/in.2016.16.6.344. Epub 2016 Dec 22. Immune Netw. 2016. PMID: 28035210 Free PMC article.
-
Active Hexose-Correlated Compound Restores Gene Expression and Protein Secretion of Protective Cytokines of Immune Cells in a Murine Stress Model during Chlamydia muridarum Genital Infection.Infect Immun. 2021 Apr 16;89(5):e00786-20. doi: 10.1128/IAI.00786-20. Print 2021 Apr 16. Infect Immun. 2021. PMID: 33558321 Free PMC article.
-
Exogenous Semaphorin 3E treatment protects against chlamydial lung infection in mice.Front Immunol. 2022 Aug 2;13:882412. doi: 10.3389/fimmu.2022.882412. eCollection 2022. Front Immunol. 2022. PMID: 35983029 Free PMC article.
References
-
- Brunham RC, Rey‐Ladino J. Immunology of Chlamydia infection: implications for a Chlamydia trachomatis vaccine. Nat Rev Immunol. 2005; 5: 149–61. - PubMed
-
- Frommell GT, Bruhn FW, Schwartzman JD. Isolation of Chlamydia trachomatis from infant lung tissue. N Engl J Med. 1977; 296: 1150–2. - PubMed
-
- Kurz H, Gopfrich H, Wabnegger L, et al Role of Chlamydophila pneumoniae in children hospitalized for community‐acquired pneumonia in Vienna, Austria. Pediatr Pulmonol. 2009; 44: 873–6. - PubMed
-
- Li W, Murthy AK, Guentzel MN, et al Antigen‐specific CD4+ T cells produce sufficient IFN‐gamma to mediate robust protective immunity against genital Chlamydia muridarum infection. J Immunol. 2008; 180: 3375–82. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

