T cell receptor signalling in the control of regulatory T cell differentiation and function
- PMID: 27026074
- PMCID: PMC4968889
- DOI: 10.1038/nri.2016.26
T cell receptor signalling in the control of regulatory T cell differentiation and function
Abstract
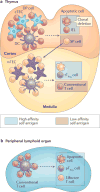
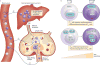
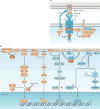
Regulatory T cells (TReg cells), a specialized T cell lineage, have a pivotal function in the control of self tolerance and inflammatory responses. Recent studies have revealed a discrete mode of T cell receptor (TCR) signalling that regulates TReg cell differentiation, maintenance and function and that affects gene expression, metabolism, cell adhesion and migration of these cells. Here, we discuss the emerging understanding of TCR-guided differentiation of TReg cells in the context of their function in health and disease.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Regulatory T cells: roles of T cell receptor for their development and function.Semin Immunopathol. 2010 Jun;32(2):95-106. doi: 10.1007/s00281-010-0200-5. Epub 2010 Feb 24. Semin Immunopathol. 2010. PMID: 20179931 Review.
-
Linked T cell receptor and cytokine signaling govern the development of the regulatory T cell repertoire.Immunity. 2008 Jan;28(1):112-21. doi: 10.1016/j.immuni.2007.11.022. Immunity. 2008. PMID: 18199418 Free PMC article.
-
Dendritic Cells Coordinate the Development and Homeostasis of Organ-Specific Regulatory T Cells.Immunity. 2016 Apr 19;44(4):847-59. doi: 10.1016/j.immuni.2016.01.025. Epub 2016 Mar 29. Immunity. 2016. PMID: 27037189 Free PMC article.
-
A two-step process for thymic regulatory T cell development.Immunity. 2008 Jan;28(1):100-11. doi: 10.1016/j.immuni.2007.11.021. Immunity. 2008. PMID: 18199417 Free PMC article.
-
Thymic and peripheral differentiation of regulatory T cells.Adv Immunol. 2011;112:25-71. doi: 10.1016/B978-0-12-387827-4.00002-4. Adv Immunol. 2011. PMID: 22118406 Review.
Cited by
-
Impact of NSCLC metabolic remodeling on immunotherapy effectiveness.Biomark Res. 2022 Aug 29;10(1):66. doi: 10.1186/s40364-022-00412-1. Biomark Res. 2022. PMID: 36038935 Free PMC article. Review.
-
Treatment with a Lactococcus lactis that chromosomally express E. coli cfaI mitigates salivary flow loss in a Sjögren's syndrome-like disease.Sci Rep. 2023 Nov 9;13(1):19489. doi: 10.1038/s41598-023-46557-3. Sci Rep. 2023. PMID: 37945636 Free PMC article.
-
Antigen exposure shapes the ratio between antigen-specific Tregs and conventional T cells in human peripheral blood.Proc Natl Acad Sci U S A. 2016 Oct 11;113(41):E6192-E6198. doi: 10.1073/pnas.1611723113. Epub 2016 Sep 28. Proc Natl Acad Sci U S A. 2016. PMID: 27681619 Free PMC article.
-
Regulatory T cells in tumor microenvironment: new mechanisms, potential therapeutic strategies and future prospects.Mol Cancer. 2020 Jul 17;19(1):116. doi: 10.1186/s12943-020-01234-1. Mol Cancer. 2020. PMID: 32680511 Free PMC article. Review.
-
Regulatory T Cells in Ovarian Carcinogenesis and Future Therapeutic Opportunities.Cancers (Basel). 2022 Nov 8;14(22):5488. doi: 10.3390/cancers14225488. Cancers (Basel). 2022. PMID: 36428581 Free PMC article. Review.
References
-
- Burnet FM. The Clonal Selection Theory of Acquired Immunity. Vanderbilt University Press; Nashville, TN: 1959.
-
- Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T Cells and Immune Tolerance. Cell. 2008;133:775–787. - PubMed
-
- Nishizuka Y. Thymus and Reproduction: Sex-linked dysgnesia of the gonad after neonatal thymectomy in mice. Science. 1969;166:753–55. - PubMed
-
- Sakaguchi S, Fukuma K, Kuribayashi K, Masuda T. Organ-specific autoimmune diseases induced in mice by elimination of T cell subset. I. Evidence for the active participation of T cells in natural self-tolerance; deficit of a T cell subset as a possible cause of autoimmune disease. J Exp Med. 1985;161:72–87. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

