Molecular and Phenotypic Characterization of a Highly Evolved Type 2 Vaccine-Derived Poliovirus Isolated from Seawater in Brazil, 2014
- PMID: 27019095
- PMCID: PMC4809597
- DOI: 10.1371/journal.pone.0152251
Molecular and Phenotypic Characterization of a Highly Evolved Type 2 Vaccine-Derived Poliovirus Isolated from Seawater in Brazil, 2014
Abstract
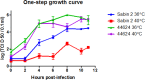
A type 2 vaccine-derived poliovirus (VDPV), differing from the Sabin 2 strain at 8.6% (78/903) of VP1 nucleotide positions, was isolated from seawater collected from a seaport in São Paulo State, Brazil. The P1/capsid region is related to the Sabin 2 strain, but sequences within the 5'-untranslated region and downstream of the P1 region were derived from recombination with other members of Human Enterovirus Species C (HEV-C). The two known attenuating mutations had reverted to wild-type (A481G in the 5'-UTR and Ile143Thr in VP1). The VDPV isolate had lost the temperature sensitive phenotype and had accumulated amino acid substitutions in neutralizing antigenic (NAg) sites 3a and 3b. The date of the initiating OPV dose, estimated from the number of synonymous substitutions in the capsid region, was approximately 8.5 years before seawater sampling, a finding consistent with a long time of virus replication and possible transmission among several individuals. Although no closely related type 2 VDPVs were detected in Brazil or elsewhere, this VDPV was found in an area with a mobile population, where conditions may favor both viral infection and spread. Environmental surveillance serves as an important tool for sensitive and early detection of circulating poliovirus in the final stages of global polio eradication.
Conflict of interest statement
Figures



Similar articles
-
Isolation of recombinant type 2 vaccine-derived poliovirus (VDPV) from a Nigerian child.Virus Res. 2007 Jul;127(1):17-25. doi: 10.1016/j.virusres.2007.03.009. Epub 2007 Apr 20. Virus Res. 2007. PMID: 17449127
-
Molecular and antigenic characterization of a highly evolved derivative of the type 2 oral poliovaccine strain isolated from sewage in Israel.J Clin Microbiol. 2000 Oct;38(10):3729-34. doi: 10.1128/JCM.38.10.3729-3734.2000. J Clin Microbiol. 2000. PMID: 11015392 Free PMC article.
-
[Genetic Characteristics of Type 2 Vaccine-derived Poliovirus in Shanxi Province (China) in 2014].Bing Du Xue Bao. 2015 Mar;31(2):157-63. Bing Du Xue Bao. 2015. PMID: 26164941 Chinese.
-
Isolation of a recombinant type 3/type 2 poliovirus with a chimeric capsid VP1 from sewage in Shandong, China.Virus Res. 2010 Jun;150(1-2):56-60. doi: 10.1016/j.virusres.2010.02.014. Epub 2010 Mar 3. Virus Res. 2010. PMID: 20206214
-
Sabin Vaccine Reversion in the Field: a Comprehensive Analysis of Sabin-Like Poliovirus Isolates in Nigeria.J Virol. 2015 Oct 14;90(1):317-31. doi: 10.1128/JVI.01532-15. Print 2016 Jan 1. J Virol. 2015. PMID: 26468545 Free PMC article.
Cited by
-
Environmental surveillance for Salmonella Typhi in rivers and wastewater from an informal sewage network in Blantyre, Malawi.PLoS Negl Trop Dis. 2024 Sep 27;18(9):e0012518. doi: 10.1371/journal.pntd.0012518. eCollection 2024 Sep. PLoS Negl Trop Dis. 2024. PMID: 39331692 Free PMC article.
-
Wastewater surveillance for viral pathogens: A tool for public health.Heliyon. 2024 Jun 29;10(13):e33873. doi: 10.1016/j.heliyon.2024.e33873. eCollection 2024 Jul 15. Heliyon. 2024. PMID: 39071684 Free PMC article. Review.
-
From vaccine to pathogen: Modeling Sabin 2 vaccine virus reversion and evolutionary epidemiology in Matlab, Bangladesh.Virus Evol. 2023 Jul 8;9(2):vead044. doi: 10.1093/ve/vead044. eCollection 2023. Virus Evol. 2023. PMID: 37692896 Free PMC article.
-
Passive sampling to scale wastewater surveillance of infectious disease: Lessons learned from COVID-19.Sci Total Environ. 2022 Aug 20;835:155347. doi: 10.1016/j.scitotenv.2022.155347. Epub 2022 Apr 20. Sci Total Environ. 2022. PMID: 35460780 Free PMC article. Review.
-
Pediatric case with vaccine-related poliovirus infection: A case report.World J Clin Pediatr. 2021 Sep 9;10(5):106-111. doi: 10.5409/wjcp.v10.i5.106. eCollection 2021 Sep 9. World J Clin Pediatr. 2021. PMID: 34616652 Free PMC article.
References
-
- Kew OM, Sutter RW, de Gourville EM, Dowdle WR, Pallansch MA. Vaccine-derived polioviruses and the endgame strategy for global polio eradication. Annu Rev Microbiol 2005. October;59:587–635. - PubMed
-
- Sutter RW, Kew OM, Cochi SL, Aylward RB. Poliovirus vaccine–live In: Plotkin SA, Ornstein WA, Offit PA, editors. Vaccines. London: W. B. Saunders; 2013. p. 598–645.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

