Plakophilin-1, a Novel Wnt Signaling Regulator, Is Critical for Tooth Development and Ameloblast Differentiation
- PMID: 27015268
- PMCID: PMC4806907
- DOI: 10.1371/journal.pone.0152206
Plakophilin-1, a Novel Wnt Signaling Regulator, Is Critical for Tooth Development and Ameloblast Differentiation
Abstract
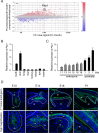
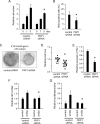
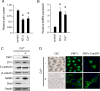
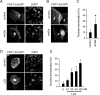
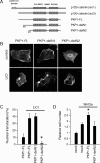
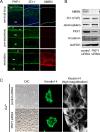
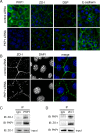
Tooth morphogenesis is initiated by reciprocal interactions between the ectoderm and neural crest-derived mesenchyme, and the Wnt signaling pathway is involved in this process. We found that Plakophilin (PKP)1, which is associated with diseases such as ectodermal dysplasia/skin fragility syndrome, was highly expressed in teeth and skin, and was upregulated during tooth development. We hypothesized that PKP1 regulates Wnt signaling via its armadillo repeat domain in a manner similar to β-catenin. To determine its role in tooth development, we performed Pkp1 knockdown experiments using ex vivo organ cultures and cell cultures. Loss of Pkp1 reduced the size of tooth germs and inhibited dental epithelial cell proliferation, which was stimulated by Wnt3a. Furthermore, transfected PKP1-emerald green fluorescent protein was translocated from the plasma membrane to the nucleus upon stimulation with Wnt3a and LiCl, which required the PKP1 N terminus (amino acids 161 to 270). Localization of PKP1, which is known as an adhesion-related desmosome component, shifted to the plasma membrane during ameloblast differentiation. In addition, Pkp1 knockdown disrupted the localization of Zona occludens 1 in tight junctions and inhibited ameloblast differentiation; the two proteins were shown to directly interact by immunoprecipitation. These results implicate the participation of PKP1 in early tooth morphogenesis as an effector of canonical Wnt signaling that controls ameloblast differentiation via regulation of the cell adhesion complex.
Conflict of interest statement
Figures
Similar articles
-
Epiprofin/Sp6 regulates Wnt-BMP signaling and the establishment of cellular junctions during the bell stage of tooth development.Cell Tissue Res. 2012 Oct;350(1):95-107. doi: 10.1007/s00441-012-1459-8. Epub 2012 Aug 7. Cell Tissue Res. 2012. PMID: 22868911
-
Enhanced Wnt/β-catenin signalling during tooth morphogenesis impedes cell differentiation and leads to alterations in the structure and mineralisation of the adult tooth.Biol Cell. 2012 Oct;104(10):603-17. doi: 10.1111/boc.201100075. Epub 2012 Aug 23. Biol Cell. 2012. PMID: 22671936
-
Spatiotemporal Expression of Wnt/β-catenin Signaling during Morphogenesis and Odontogenesis of Deciduous Molar in Miniature Pig.Int J Biol Sci. 2017 Sep 3;13(8):1082-1091. doi: 10.7150/ijbs.20905. eCollection 2017. Int J Biol Sci. 2017. PMID: 28924388 Free PMC article.
-
Plakophilin 1 in carcinogenesis.Mol Carcinog. 2024 Oct;63(10):1855-1865. doi: 10.1002/mc.23779. Epub 2024 Jun 18. Mol Carcinog. 2024. PMID: 38888207 Review.
-
Ectodermal dysplasia-skin fragility syndrome.Dermatol Clin. 2010 Jan;28(1):125-9. doi: 10.1016/j.det.2009.10.014. Dermatol Clin. 2010. PMID: 19945625 Review.
Cited by
-
The transcription factor NKX2-3 mediates p21 expression and ectodysplasin-A signaling in the enamel knot for cusp formation in tooth development.J Biol Chem. 2018 Sep 21;293(38):14572-14584. doi: 10.1074/jbc.RA118.003373. Epub 2018 Aug 8. J Biol Chem. 2018. PMID: 30089653 Free PMC article.
-
Development of a novel ex vivo organ culture system to improve preservation methods of regenerative tissues.Sci Rep. 2023 Feb 27;13(1):3354. doi: 10.1038/s41598-023-29629-2. Sci Rep. 2023. PMID: 36849572 Free PMC article.
-
Desmosomes as Signaling Hubs in the Regulation of Cell Behavior.Front Cell Dev Biol. 2021 Sep 23;9:745670. doi: 10.3389/fcell.2021.745670. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34631720 Free PMC article. Review.
-
Expression Patterns of Claudin Family Members During Tooth Development and the Role of Claudin-10 (Cldn10) in Cytodifferentiation of Stratum Intermedium.Front Cell Dev Biol. 2020 Oct 22;8:595593. doi: 10.3389/fcell.2020.595593. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33195274 Free PMC article.
-
Single-Cell RNA-Sequencing From Mouse Incisor Reveals Dental Epithelial Cell-Type Specific Genes.Front Cell Dev Biol. 2020 Sep 1;8:841. doi: 10.3389/fcell.2020.00841. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32984333 Free PMC article.
References
-
- Lesot H, Kieffer-Combeau S, Fausser JL, Meyer JM, Perrin-Schmitt F, Peterkova R, et al. Cell-cell and cell-matrix interactions during initial enamel organ histomorphogenesis in the mouse. Connect Tissue Res. 2002;43(2–3):191–200. Epub 2002/12/20. . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

