Single Low Dose Primaquine (0.25 mg/kg) Does Not Cause Clinically Significant Haemolysis in G6PD Deficient Subjects
- PMID: 27010542
- PMCID: PMC4807095
- DOI: 10.1371/journal.pone.0151898
Single Low Dose Primaquine (0.25 mg/kg) Does Not Cause Clinically Significant Haemolysis in G6PD Deficient Subjects
Abstract
Background: Primaquine is the only drug consistently effective against mature gametocytes of Plasmodium falciparum. The transmission blocking dose of primaquine previously recommended was 0.75 mg/kg (adult dose 45 mg) but its deployment was limited because of concerns over haemolytic effects in patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency. G6PD deficiency is an inherited X-linked enzymatic defect that affects an estimated 400 million people around the world with high frequencies (15-20%) in populations living in malarious areas. To reduce transmission in low transmission settings and facilitate elimination of P. falciparum, the World Health Organization now recommends adding a single dose of 0.25 mg/kg (adult dose 15 mg) to Artemisinin-based Combination Therapies (ACTs) without G6PD testing. Direct evidence of the safety of this low dose is lacking. Adverse events and haemoglobin variations after this treatment were assessed in both G6PD normal and deficient subjects in the context of targeted malaria elimination in a malaria endemic area on the North-Western Myanmar-Thailand border where prevalence of G6PD deficiency (Mahidol variant) approximates 15%.
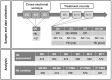
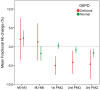
Methods and findings: The tolerability and safety of primaquine (single dose 0.25 mg base/kg) combined with dihydroartemisinin-piperaquine (DHA-PPQ) given three times at monthly intervals was assessed in 819 subjects. Haemoglobin concentrations were estimated over the six months preceding the ACT + primaquine rounds of mass drug administration. G6PD deficiency was assessed with a phenotypic test and genotyping was performed in male subjects with deficient phenotypes and in all females. Fractional haemoglobin changes in relation to G6PD phenotype and genotype and primaquine round were assessed using linear mixed-effects models. No adverse events related to primaquine were reported during the trial. Mean fractional haemoglobin changes after each primaquine treatment in G6PD deficient subjects (-5.0%, -4.2% and -4.7%) were greater than in G6PD normal subjects (0.3%, -0.8 and -1.7%) but were clinically insignificant. Fractional drops in haemoglobin concentration larger than 25% following single dose primaquine were observed in 1.8% of the population but were asymptomatic.
Conclusions: The single low dose (0.25mg/kg) of primaquine is clinically well tolerated and can be used safely without prior G6PD testing in populations with high prevalence of G6PD deficiency. The present evidence supports a broader use of low dose primaquine without G6PD testing for the treatment and elimination of falciparum malaria.
Trial registration: ClinicalTrials.gov NCT01872702.
Conflict of interest statement
Figures
Similar articles
-
Primaquine for reducing Plasmodium falciparum transmission.Cochrane Database Syst Rev. 2012 Sep 12;(9):CD008152. doi: 10.1002/14651858.CD008152.pub2. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2014 Jun 30;(6):CD008152. doi: 10.1002/14651858.CD008152.pub3. PMID: 22972117 Updated. Review.
-
Haemolysis in G6PD Heterozygous Females Treated with Primaquine for Plasmodium vivax Malaria: A Nested Cohort in a Trial of Radical Curative Regimens.PLoS Med. 2017 Feb 7;14(2):e1002224. doi: 10.1371/journal.pmed.1002224. eCollection 2017 Feb. PLoS Med. 2017. PMID: 28170391 Free PMC article. Clinical Trial.
-
Safety of age-dosed, single low-dose primaquine in children with glucose-6-phosphate dehydrogenase deficiency who are infected with Plasmodium falciparum in Uganda and the Democratic Republic of the Congo: a randomised, double-blind, placebo-controlled, non-inferiority trial.Lancet Infect Dis. 2023 Apr;23(4):471-483. doi: 10.1016/S1473-3099(22)00658-2. Epub 2022 Nov 30. Lancet Infect Dis. 2023. PMID: 36462528 Clinical Trial.
-
Primaquine or other 8-aminoquinoline for reducing P. falciparum transmission.Cochrane Database Syst Rev. 2014 Jun 30;(6):CD008152. doi: 10.1002/14651858.CD008152.pub3. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2015 Feb 19;(2):CD008152. doi: 10.1002/14651858.CD008152.pub4. PMID: 24979199 Free PMC article. Updated. Review.
-
Primaquine or other 8-aminoquinolines for reducing Plasmodium falciparum transmission.Cochrane Database Syst Rev. 2018 Feb 2;2(2):CD008152. doi: 10.1002/14651858.CD008152.pub5. Cochrane Database Syst Rev. 2018. PMID: 29393511 Free PMC article. Review.
Cited by
-
The medication for pneumocystis pneumonia with glucose-6-phosphate dehydrogenase deficiency patients.Front Pharmacol. 2022 Sep 7;13:957376. doi: 10.3389/fphar.2022.957376. eCollection 2022. Front Pharmacol. 2022. PMID: 36160421 Free PMC article. Review.
-
Implications of current therapeutic restrictions for primaquine and tafenoquine in the radical cure of vivax malaria.PLoS Negl Trop Dis. 2018 Apr 20;12(4):e0006440. doi: 10.1371/journal.pntd.0006440. eCollection 2018 Apr. PLoS Negl Trop Dis. 2018. PMID: 29677199 Free PMC article.
-
Plasmodium falciparum genotype and gametocyte prevalence in children with uncomplicated malaria in coastal Ghana.Malar J. 2016 Dec 9;15(1):592. doi: 10.1186/s12936-016-1640-8. Malar J. 2016. PMID: 27938356 Free PMC article.
-
An optimised age-based dosing regimen for single low-dose primaquine for blocking malaria transmission in Cambodia.BMC Med. 2016 Oct 27;14(1):171. doi: 10.1186/s12916-016-0701-8. BMC Med. 2016. PMID: 27784313 Free PMC article.
-
Factors affecting haemoglobin dynamics in African children with acute uncomplicated Plasmodium falciparum malaria treated with single low-dose primaquine or placebo.BMC Med. 2023 Oct 20;21(1):397. doi: 10.1186/s12916-023-03105-0. BMC Med. 2023. PMID: 37858129 Free PMC article. Clinical Trial.
References
-
- WHO ERG. The Safety and Effectiveness of Single Dose Primaquine as a P. falciparum gametocytocide 2012. Available: http://www.who.int/malaria/mpac/sep2012/primaquine_single_dose_pf_erg_me....
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

