The altered glucose metabolism in tumor and a tumor acidic microenvironment associated with extracellular matrix metalloproteinase inducer and monocarboxylate transporters
- PMID: 27009812
- PMCID: PMC5029616
- DOI: 10.18632/oncotarget.8153
The altered glucose metabolism in tumor and a tumor acidic microenvironment associated with extracellular matrix metalloproteinase inducer and monocarboxylate transporters
Abstract
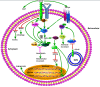
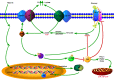
Extracellular matrix metalloproteinase inducer, also knowns as cluster of differentiation 147 (CD147) or basigin, is a widely distributed cell surface glycoprotein that is involved in numerous physiological and pathological functions, especially in tumor invasion and metastasis. Monocarboxylate transporters (MCTs) catalyze the proton-linked transport of monocarboxylates such as L-lactate across the plasma membrane to preserve the intracellular pH and maintain cell homeostasis. As a chaperone to some MCT isoforms, CD147 overexpression significantly contributes to the metabolic transformation of tumor. This overexpression is characterized by accelerated aerobic glycolysis and lactate efflux, and it eventually provides the tumor cells with a metabolic advantage and an invasive phenotype in the acidic tumor microenvironment. This review highlights the roles of CD147 and MCTs in tumor cell metabolism and the associated molecular mechanisms. The regulation of CD147 and MCTs may prove to be with a therapeutic potential for tumors through the metabolic modification of the tumor microenvironment.
Keywords: extracellular matrix metalloproteinase inducer; glycolysis; monocarboxylate transporters; p53; tumor acidic microenvironment.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Tumor metabolism of lactate: the influence and therapeutic potential for MCT and CD147 regulation.Future Oncol. 2010 Jan;6(1):127-48. doi: 10.2217/fon.09.145. Future Oncol. 2010. PMID: 20021214 Free PMC article. Review.
-
MCT1, MCT4 and CD147 expression and 3-bromopyruvate toxicity in colorectal cancer cells are modulated by the extracellular conditions.Biol Chem. 2019 May 27;400(6):787-799. doi: 10.1515/hsz-2018-0411. Biol Chem. 2019. PMID: 30699066
-
Knock out of the BASIGIN/CD147 chaperone of lactate/H+ symporters disproves its pro-tumour action via extracellular matrix metalloproteases (MMPs) induction.Oncotarget. 2015 Sep 22;6(28):24636-48. doi: 10.18632/oncotarget.4323. Oncotarget. 2015. PMID: 26284589 Free PMC article.
-
CD147/Basigin Is Involved in the Development of Malignant Tumors and T-Cell-Mediated Immunological Disorders via Regulation of Glycolysis.Int J Mol Sci. 2023 Dec 11;24(24):17344. doi: 10.3390/ijms242417344. Int J Mol Sci. 2023. PMID: 38139173 Free PMC article. Review.
-
Monocarboxylate transporters 1 and 4 are associated with CD147 in cervical carcinoma.Dis Markers. 2009;26(3):97-103. doi: 10.3233/DMA-2009-0596. Dis Markers. 2009. PMID: 19597291 Free PMC article.
Cited by
-
Competitive glucose metabolism as a target to boost bladder cancer immunotherapy.Nat Rev Urol. 2020 Feb;17(2):77-106. doi: 10.1038/s41585-019-0263-6. Epub 2020 Jan 17. Nat Rev Urol. 2020. PMID: 31953517 Review.
-
[Effect of Fei-Liu-Ping ointment combined with cyclophosphamide on lung cancer cell proliferation and acidic microenvironment].Beijing Da Xue Xue Bao Yi Xue Ban. 2020 Apr 18;52(2):247-253. doi: 10.19723/j.issn.1671-167X.2020.02.009. Beijing Da Xue Xue Bao Yi Xue Ban. 2020. PMID: 32306006 Free PMC article. Chinese.
-
Correlation between 18F-FDG maximum standardized uptake value with CD147 expression in lung adenocarcinomas: a retrospective study.PeerJ. 2019 Sep 9;7:e7635. doi: 10.7717/peerj.7635. eCollection 2019. PeerJ. 2019. PMID: 31565568 Free PMC article.
-
A Root in Synapsis and the Other One in the Gut Microbiome-Brain Axis: Are the Two Poles of Ketogenic Diet Enough to Challenge Glioblastoma?Front Nutr. 2021 Jul 22;8:703392. doi: 10.3389/fnut.2021.703392. eCollection 2021. Front Nutr. 2021. PMID: 34422883 Free PMC article. Review.
-
Glucose Promotes EMMPRIN/CD147 and the Secretion of Pro-Angiogenic Factors in a Co-Culture System of Endothelial Cells and Monocytes.Biomedicines. 2024 Mar 22;12(4):706. doi: 10.3390/biomedicines12040706. Biomedicines. 2024. PMID: 38672062 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

