Norovirus antagonism of B-cell antigen presentation results in impaired control of acute infection
- PMID: 27007673
- PMCID: PMC5035161
- DOI: 10.1038/mi.2016.15
Norovirus antagonism of B-cell antigen presentation results in impaired control of acute infection
Abstract
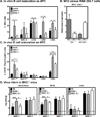
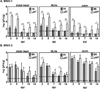
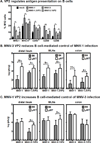
Human noroviruses are a leading cause of gastroenteritis, and so, vaccine development is desperately needed. Elucidating viral mechanisms of immune antagonism can provide key insight into designing effective immunization platforms. We recently revealed that B cells are targets of norovirus infection. Because noroviruses can regulate antigen presentation by infected macrophages and B cells can function as antigen-presenting cells, we tested whether noroviruses regulate B-cell-mediated antigen presentation and the biological consequence of such regulation. Indeed, murine noroviruses could prevent B-cell expression of antigen presentation molecules and this directly correlated with impaired control of acute infection. In addition to B cells, acute control required MHC class I molecules, CD8+ T cells, and granzymes, supporting a model whereby B cells act as antigen presenting cells to activate cytotoxic CD8+ T cells. This immune pathway was active prior to the induction of antiviral antibody responses. As in macrophages, the minor structural protein VP2 regulated B-cell antigen presentation in a virus-specific manner. Commensal bacteria were not required for the activation of this pathway and ultimately only B cells were required for the clearance of viral infection. These findings provide new insight into the role of B cells in stimulating antiviral CD8+ T-cell responses.
Figures



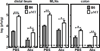
Similar articles
-
Mouse Norovirus Infection Reduces the Surface Expression of Major Histocompatibility Complex Class I Proteins and Inhibits CD8+ T Cell Recognition and Activation.J Virol. 2018 Aug 29;92(18):e00286-18. doi: 10.1128/JVI.00286-18. Print 2018 Sep 15. J Virol. 2018. PMID: 29976673 Free PMC article.
-
Norovirus mechanisms of immune antagonism.Curr Opin Virol. 2016 Feb;16:24-30. doi: 10.1016/j.coviro.2015.11.005. Epub 2015 Dec 7. Curr Opin Virol. 2016. PMID: 26673810 Free PMC article. Review.
-
Identification of immune and viral correlates of norovirus protective immunity through comparative study of intra-cluster norovirus strains.PLoS Pathog. 2013;9(9):e1003592. doi: 10.1371/journal.ppat.1003592. Epub 2013 Sep 5. PLoS Pathog. 2013. PMID: 24039576 Free PMC article.
-
Regulation of Norovirus Virulence by the VP1 Protruding Domain Correlates with B Cell Infection Efficiency.J Virol. 2015 Dec 30;90(6):2858-67. doi: 10.1128/JVI.02880-15. J Virol. 2015. PMID: 26719276 Free PMC article.
-
Viral shape-shifting: norovirus evasion of the human immune system.Nat Rev Microbiol. 2010 Mar;8(3):231-41. doi: 10.1038/nrmicro2296. Epub 2010 Feb 2. Nat Rev Microbiol. 2010. PMID: 20125087 Free PMC article. Review.
Cited by
-
Immunity to enteric viruses.Immunity. 2022 May 10;55(5):800-818. doi: 10.1016/j.immuni.2022.04.007. Immunity. 2022. PMID: 35545029 Free PMC article. Review.
-
Antibody Production Remains Intact Despite Loss of Bone Marrow B cells in Murine Norovirus Infected Stat1-/- Mice.Comp Med. 2021 Dec 1;71(6):502-511. doi: 10.30802/AALAS-CM-21-000054. Epub 2021 Nov 18. Comp Med. 2021. PMID: 34794531 Free PMC article.
-
Linear epitopes on the capsid protein of norovirus commonly elicit high antibody response among past-infected individuals.Virol J. 2023 Jun 6;20(1):115. doi: 10.1186/s12985-023-02087-y. Virol J. 2023. PMID: 37280660 Free PMC article.
-
Murine norovirus inhibits B cell development in the bone marrow of STAT1-deficient mice.Virology. 2018 Feb;515:123-133. doi: 10.1016/j.virol.2017.12.013. Epub 2017 Dec 26. Virology. 2018. PMID: 29287229 Free PMC article.
-
Interferon responses to norovirus infections: current and future perspectives.J Gen Virol. 2021 Oct;102(10):001660. doi: 10.1099/jgv.0.001660. J Gen Virol. 2021. PMID: 34698626 Free PMC article. Review.
References
-
- CDC - 2011 Estimates of Foodborne Illness [Internet] 2011 Available from: http://www.cdc.gov/foodborneburden/2011-foodborne-estimates.html.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

