Artificial antigen-presenting cells expressing AFP(158-166) peptide and interleukin-15 activate AFP-specific cytotoxic T lymphocytes
- PMID: 27007051
- PMCID: PMC4951234
- DOI: 10.18632/oncotarget.8198
Artificial antigen-presenting cells expressing AFP(158-166) peptide and interleukin-15 activate AFP-specific cytotoxic T lymphocytes
Abstract
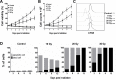
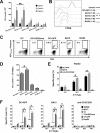
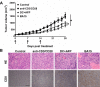
Professional antigen-presenting cells (APCs) are potent generators of tumor antigen-specific cytotoxic T lymphocytes (CTLs) for adoptive immunotherapy; however, generation of APCs is cumbersome, expensive, and subject to the tumor microenvironment. Artificial APCs (aAPCs) have been developed as a cost-effective alternative to APCs. We developed a cellular aAPC that efficiently generated alpha-fetoprotein (AFP)-specific CTLs. We genetically modified the human B cell lymphoma cell line BJAB with a lentiviral vector to establish an aAPC called BA15. The expression of AFP(158-166)-HLA-A*02:01 complex, CD80, CD86, and interleukin (IL)-15 in BA15 cells was assessed. The efficiency of BA15 at generating AFP-specific CTLs and the specific cytotoxicity of CTLs against AFP+ cells were also determined. BA15 cells expressed high levels of AFP(158-166) peptide, HLA-A2, CD80, CD86, and IL-15. BA15 cells also exhibited higher efficiency in generating AFP-specific CTLs than did dendritic cells. These CTLs had greater cytotoxicity against AFP+ hepatocellular carcinoma cells than did CTLs obtained from dendritic cells in vitro and in vivo. Our novel aAPC system could provide a robust platform for the generation of functional AFP-specific CTLs for adoptive immunotherapy of hepatocellular carcinoma.
Keywords: Immune response; Immunity; Immunology and Microbiology Section; adoptive immunotherapy; alpha-fetoprotein; artificial antigen-presenting cells; cytotoxic T lymphocytes; interleukin-15.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Engineered cytotoxic T lymphocytes with AFP-specific TCR gene for adoptive immunotherapy in hepatocellular carcinoma.Tumour Biol. 2016 Jan;37(1):799-806. doi: 10.1007/s13277-015-3845-9. Epub 2015 Aug 7. Tumour Biol. 2016. PMID: 26250457
-
Artificial antigen-presenting cells transduced with telomerase efficiently expand epitope-specific, human leukocyte antigen-restricted cytotoxic T cells.Cancer Res. 2005 Jun 15;65(12):5417-27. doi: 10.1158/0008-5472.CAN-04-2991. Cancer Res. 2005. PMID: 15958591
-
Programmed Death-Ligand 1 on Antigen-presenting Cells Facilitates the Induction of Antigen-specific Cytotoxic T Lymphocytes: Application to Adoptive T-Cell Immunotherapy.J Immunother. 2016 Oct;39(8):306-15. doi: 10.1097/CJI.0000000000000136. J Immunother. 2016. PMID: 27548033
-
Immunotherapeutic strategies for hepatocellular carcinoma.Gastroenterology. 2004 Nov;127(5 Suppl 1):S232-41. doi: 10.1053/j.gastro.2004.09.038. Gastroenterology. 2004. PMID: 15508089 Review.
-
Alpha-Fetoprotein and Hepatocellular Carcinoma Immunity.Can J Gastroenterol Hepatol. 2018 Apr 1;2018:9049252. doi: 10.1155/2018/9049252. eCollection 2018. Can J Gastroenterol Hepatol. 2018. PMID: 29805966 Free PMC article. Review.
Cited by
-
Paracrine release of IL-2 and anti-CTLA-4 enhances the ability of artificial polymer antigen-presenting cells to expand antigen-specific T cells and inhibit tumor growth in a mouse model.Cancer Immunol Immunother. 2017 Sep;66(9):1229-1241. doi: 10.1007/s00262-017-2016-9. Epub 2017 May 13. Cancer Immunol Immunother. 2017. PMID: 28501941 Free PMC article.
-
Chemically Engineered Immune Cell-Derived Microrobots and Biomimetic Nanoparticles: Emerging Biodiagnostic and Therapeutic Tools.Adv Sci (Weinh). 2021 Mar 1;8(8):2002499. doi: 10.1002/advs.202002499. eCollection 2021 Apr. Adv Sci (Weinh). 2021. PMID: 33898169 Free PMC article. Review.
-
The Role of Alpha-Fetoprotein (AFP) in Contemporary Oncology: The Path from a Diagnostic Biomarker to an Anticancer Drug.Int J Mol Sci. 2023 Jan 28;24(3):2539. doi: 10.3390/ijms24032539. Int J Mol Sci. 2023. PMID: 36768863 Free PMC article. Review.
References
-
- Ma C, Cheung AF, Chodon T, Koya RC, Wu Z, Ng C, Avramis E, Cochran AJ, Witte ON, Baltimore D, Chmielowski B, Economou JS, Comin-Anduix B, Ribas A, Heath JR. Multifunctional T-cell analyses to study response and progression in adoptive cell transfer immunotherapy. Cancer Discov. 2013;3:418–429. - PMC - PubMed
-
- Schuessler A, Smith C, Beagley L, Boyle GM, Rehan S, Matthews K, Jones L, Crough T, Dasari V, Klein K, Smalley A, Alexander H, Walker DG, Khanna R. Autologous T-cell therapy for cytomegalovirus as a consolidative treatment for recurrent glioblastoma. Cancer Res. 2014;74:3466–3476. - PubMed
-
- Martelli MF, Di Ianni M, Ruggeri L, Falzetti F, Carotti A, Terenzi A, Pierini A, Massei MS, Amico L, Urbani E, Del Papa B, Zei T, Iacucci Ostini R, Cecchini D, Tognellini R, Reisner Y, Aversa F, Falini B, Velardi A. HLA-haploidentical transplantation with regulatory and conventional T-cell adoptive immunotherapy prevents acute leukemia relapse. Blood. 2014;124:638–644. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

