Teriflunomide for multiple sclerosis
- PMID: 27003123
- PMCID: PMC10493042
- DOI: 10.1002/14651858.CD009882.pub3
Teriflunomide for multiple sclerosis
Abstract
Background: This is an update of the Cochrane review "Teriflunomide for multiple sclerosis" (first published in The Cochrane Library 2012, Issue 12).Multiple sclerosis (MS) is a chronic immune-mediated disease of the central nervous system. It is clinically characterized by recurrent relapses or progression, or both, often leading to severe neurological disability and a serious decline in quality of life. Disease-modifying therapies (DMTs) for MS aim to prevent occurrence of relapses and disability progression. Teriflunomide is a pyrimidine synthesis inhibitor approved by both the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) as a DMT for adults with relapsing-remitting MS (RRMS).
Objectives: To assess the absolute and comparative effectiveness and safety of teriflunomide as monotherapy or combination therapy versus placebo or other disease-modifying drugs (DMDs) (interferon beta (IFNβ), glatiramer acetate, natalizumab, mitoxantrone, fingolimod, dimethyl fumarate, alemtuzumab) for modifying the disease course in people with MS.
Search methods: We searched the Cochrane Multiple Sclerosis and Rare Diseases of the CNS Group Specialised Trials Register (30 September 2015). We checked reference lists of published reviews and retrieved articles and searched reports (2004 to September 2015) from the MS societies in Europe and America. We also communicated with investigators participating in trials of teriflunomide and the pharmaceutical company, Sanofi-Aventis.
Selection criteria: We included randomized, controlled, parallel-group clinical trials with a length of follow-up of one year or greater evaluating teriflunomide, as monotherapy or combination therapy, versus placebo or other approved DMDs for people with MS without restrictions regarding dose, administration frequency and duration of treatment.
Data collection and analysis: We used the standard methodological procedures of Cochrane. Two review authors independently assessed trial quality and extracted data. Disagreements were discussed and resolved by consensus among the review authors. We contacted the principal investigators of included studies for additional data or confirmation of data.
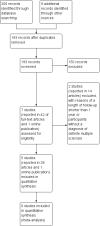
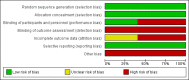
Main results: Five studies involving 3231 people evaluated the efficacy and safety of teriflunomide 7 mg and 14 mg, alone or with add-on IFNβ, versus placebo or IFNβ-1a for adults with relapsing forms of MS and an entry Expanded Disability Status Scale score of less than 5.5.Overall, there were obvious clinical heterogeneities due to diversities in study designs or interventions and methodological heterogeneities across studies. All studies had a high risk of detection bias for relapse assessment and a high risk of bias due to conflicts of interest. Among them, three studies additionally had a high risk of attrition bias due to a high dropout rate and two studies had an unclear risk of attrition bias. The studies of combination therapy with IFNβ (650 participants) and the study with IFNβ-1a as controls (324 participants) also had a high risk for performance bias and a lack of power due to the limited sample.Two studies evaluated the benefit and the safety of teriflunomide as monotherapy versus placebo over a period of one year (1169 participants) or two years (1088 participants). A meta-analysis was not conducted. Compared to placebo, administration of teriflunomide at a dose of 7 mg/day or 14 mg/day as monotherapy reduced the number of participants with at least one relapse over one year (risk ratio (RR) 0.72, 95% confidence interval (CI) 0.59 to 0.87, P value = 0.001 with 7 mg/day and RR 0.60, 95% CI 0.48 to 0.75, P value < 0.00001 with 14 mg/day) or two years (RR 0.85, 95% CI 0.74 to 0.98, P value = 0.03 with 7 mg/day and RR 0.80, 95% CI 0.69 to 0.93, P value = 0.004 with 14 days). Only teriflunomide at a dose of 14 mg/day reduced the number of participants with disability progression over one year (RR 0.55, 95% CI 0.36 to 0.84, P value = 0.006) or two years (RR 0.74, 95% CI 0.56 to 0.96, P value = 0.02). When taking the effect of drop-outs into consideration, the likely-case scenario analyses still showed a benefit in reducing the number of participants with at least one relapse, but not for the number of participants with disability progression. Both doses also reduced the annualized relapse rate and the number of gadolinium-enhancing T1-weighted lesions over two years. Quality of evidence for relapse outcomes at one year or at two years was low, while for disability progression at one year or at two years was very low.When compared to IFNβ-1a, teriflunomide at a dose of 14 mg/day had a similar efficacy to IFNβ-1a in reducing the proportion of participants with at least one relapse over one year, while teriflunomide at a dose of 7 mg/day was inferior to IFNβ-1a (RR 1.52, 95% CI 0.87 to 2.67, P value = 0.14; 215 participants with 14 mg/day and RR 2.74, 95% CI 1.66 to 4.53, P value < 0.0001; 213 participants with 7 mg/day). However, the quality of evidence was very low.In terms of safety profile, the most common adverse events associated with teriflunomide were diarrhoea, nausea, hair thinning, elevated alanine aminotransferase, neutropenia and lymphopenia. These adverse events had a dose-related effects and rarely led to treatment discontinuation.
Authors' conclusions: There was low-quality evidence to support that teriflunomide at a dose of 7 mg/day or 14 mg/day as monotherapy reduces both the number of participants with at least one relapse and the annualized relapse rate over one year or two years of treatment in comparison with placebo. Only teriflunomide at a dose of 14 mg/day reduced the number of participants with disability progression and delayed the progression of disability over one year or two years, but the quality of the evidence was very low. The quality of available data was too low to evaluate the benefit teriflunomide as monotherapy versus IFNβ-1a or as combination therapy with IFNβ. The common adverse effects were diarrhoea, nausea, hair thinning, elevated alanine aminotransferase, neutropenia and lymphopenia. These adverse effects were mostly mild-to-moderate in severity, but had a dose-related effect. New studies of high quality and longer follow-up are needed to evaluate the comparative benefit of teriflunomide on these outcomes and the safety in comparison with other DMTs.
Conflict of interest statement
DH ‐ none.
CZ ‐ none.
XZ ‐ none.
YZ ‐ none.
QD ‐ none.
YL ‐ none.
LC‐ none.
Figures
Update of
-
Teriflunomide for multiple sclerosis.Cochrane Database Syst Rev. 2012 Dec 12;12:CD009882. doi: 10.1002/14651858.CD009882.pub2. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2016 Mar 22;3:CD009882. doi: 10.1002/14651858.CD009882.pub3. PMID: 23235682 Updated. Review.
Similar articles
-
Teriflunomide for multiple sclerosis.Cochrane Database Syst Rev. 2012 Dec 12;12:CD009882. doi: 10.1002/14651858.CD009882.pub2. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2016 Mar 22;3:CD009882. doi: 10.1002/14651858.CD009882.pub3. PMID: 23235682 Updated. Review.
-
Dimethyl fumarate for multiple sclerosis.Cochrane Database Syst Rev. 2015 Apr 22;2015(4):CD011076. doi: 10.1002/14651858.CD011076.pub2. Cochrane Database Syst Rev. 2015. PMID: 25900414 Free PMC article. Review.
-
Immunomodulators and immunosuppressants for relapsing-remitting multiple sclerosis: a network meta-analysis.Cochrane Database Syst Rev. 2024 Jan 4;1(1):CD011381. doi: 10.1002/14651858.CD011381.pub3. Cochrane Database Syst Rev. 2024. PMID: 38174776 Free PMC article. Review.
-
Treatment with disease-modifying drugs for people with a first clinical attack suggestive of multiple sclerosis.Cochrane Database Syst Rev. 2017 Apr 25;4(4):CD012200. doi: 10.1002/14651858.CD012200.pub2. Cochrane Database Syst Rev. 2017. PMID: 28440858 Free PMC article. Review.
-
Rituximab for relapsing-remitting multiple sclerosis.Cochrane Database Syst Rev. 2013 Dec 6;(12):CD009130. doi: 10.1002/14651858.CD009130.pub3. Cochrane Database Syst Rev. 2013. PMID: 24310855 Review.
Cited by
-
A comparison of clinical, utilization, and cost outcomes between oral treatments for multiple sclerosis.J Manag Care Spec Pharm. 2024 Feb 3;30(2):129-140. doi: 10.18553/jmcp.2024.30.2.129. J Manag Care Spec Pharm. 2024. PMID: 38308623 Free PMC article.
-
Pharmacovigilance for rare diseases: a bibliometrics and knowledge-map analysis based on web of science.Orphanet J Rare Dis. 2023 Sep 26;18(1):303. doi: 10.1186/s13023-023-02915-y. Orphanet J Rare Dis. 2023. PMID: 37752556 Free PMC article. Review.
-
Family Planning in Fertile-Age Patients With Multiple Sclerosis (MS) (ConPlanEM Study): Delphi Consensus Statements.Cureus. 2023 Aug 24;15(8):e44056. doi: 10.7759/cureus.44056. eCollection 2023 Aug. Cureus. 2023. PMID: 37746391 Free PMC article.
-
Surgically Induced Demyelination in Rat Sciatic Nerve.Brain Sci. 2023 May 3;13(5):754. doi: 10.3390/brainsci13050754. Brain Sci. 2023. PMID: 37239226 Free PMC article.
-
A Dual Therapy of Nanostructured Lipid Carrier Loaded with Teriflunomide-A Dihydro-Orotate Dehydrogenase Inhibitor and an miR-155-Antagomir in Cuprizone-Induced C57BL/6J Mouse.Pharmaceutics. 2023 Apr 17;15(4):1254. doi: 10.3390/pharmaceutics15041254. Pharmaceutics. 2023. PMID: 37111739 Free PMC article.
References
References to studies included in this review
Confavreux 2014 {published data only}
-
- Comi G, Freedman MS, Kappos L, Miller A, Olsson TP, Wolinsky JS, et al. Effect of teriflunomide on lymphocyte and neutrophil counts in patients with relapsing multiple sclerosis: results from the TOWER study. Journal of the Neurological Sciences 2013;333:e376.
-
- Confavreux C, O'Connor P, Comi G, Freedman MS, Miller AE, Olsson TP, et al. Oral teriflunomide for patients with relapsing multiple sclerosis (TOWER): a randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet Neurology 2014;13(3):247‐56. - PubMed
-
- Kappos L, Comi G, Confavreux C, Freedman MS, Miller AE, Olsson TP, et al. The efficacy and safety of teriflunomide in patients with relapsing MS: results from TOWER, a phase Ⅲ, placebo‐controlled study. Multiple Sclerosis 2012;18:50‐1.
-
- Miller A, Kappos L, Comi G, Confavreux C, Freedman M, Olsson T, et al. Teriflunomide efficacy and safety in patients with relapsing multiple sclerosis: results from tower, a second, pivotal, phase 3 placebo‐controlled study. Neurology. 2013; Vol. 80.
Freedman 2012 {published data only}
-
- Freedman MS, Wolinsky JS, Wamil B, Confavreux C, Comi G, Kappos L, et al. Teriflunomide added to interferon‐β in relapsing multiple sclerosis: a randomized phase Ⅱ trial. Neurology 2012;78(23):1877‐85. - PubMed
NCT01252355 {published data only}
-
- NCT01252355. Efficacy and Safety of Teriflunomide in Patients with Relapsing Multiple Sclerosis and Treated with Interferon‐beta (TERACLES). www.clinicaltrials.gov/ct2/show/results/NCT01252355 (accessed 12 January 2016).
O'Connor 2011 {published data only}
-
- Comi G, Benzerdjeb H, Wang L, Truffinet P, O'Connor P. Effect of teriflunomide on lymphocyte and neutrophil levels in patients with relapsing multiple sclerosis: results from the TEMSO study. Journal of Neurological Science 2013;333(Suppl 1):e376.
-
- Comi G, O'Connor P, Wolinsky J, Confavreux C, Kappos L, Olsson T, et al. Extension of a phase Ⅲ trial (TEMSO) of oral teriflunomide in multiple sclerosis with relapses: safety outcomes with up to 4 years of follow‐Up. Multiple Sclerosis 2011;17:S182‐3.
-
- Freedman M, Wolinsky J, Comi G, Kappos L, Olsson T, Miller A, et al. Long‐term safety and efficacy of teriflunomide in patients with relapsing forms of multiple sclerosis in the TEMSO extension trial. Multiple Sclerosis 2013;19:225.
-
- Freedman M, Wolinsky J, Comi G, Kappos L, Olsson T, Miller A, et al. Safety and efficacy of teriflunomide for up to 9 years in relapsing forms of multiple sclerosis: Update of the TEMSO extension trial. Neurology 2014; Vol. 82, issue Suppl 10:3‐150.
-
- Freedman MS, Delhay JL, Benamor M. Gastrointestinal symptoms infrequently lead to discontinuation of teriflunomide therapy. Multiple Sclerosis 2012;18:S13.
Vermersch 2014 {published data only}
-
- Vermersch P, Czlonkowska A, Grimaldi LM, Confavreux C, Comi G, Kappos L, et al. A multicenter, randomized, parallel‐group, rater‐blinded study comparing the effectiveness and safety of teriflunomide and subcutaneous interferon beta‐1a in patients with relapsing multiple sclerosis. Multiple Sclerosis 2012;18:S9‐10.
-
- Vermersch P, Czlonkowska A, Grimaldi LM, Confavreux C, Comi G, Kappos L, et al. Evaluation of patient satisfaction from the TENERE study: a comparison of teriflunomide and subcutaneous interferon beta‐1a in patients with relapsing multiple sclerosis. Journal of Neurology 2012;259:S38.
-
- Vermersch P, Czlonkowska A, Grimaldi LM, Confavreux C, Comi G, Kappos L, et al. Teriflunomide versus subcutaneous interferon beta‐1a in patients with relapsing multiple sclerosis: a randomised, controlled phase 3 trial. Multiple Sclerosis 2014;20(6):705‐16. - PubMed
References to studies excluded from this review
Miller 2014 {published data only}
-
- Comi G, Miller AE, Wolinsky JS, Benamor M, Bauer D, Truffinet P, et al. The effect of teriflunomide on lymphocyte and neutrophil count in patients with a first clinical episode consistent with multiple sclerosis: results from the TOPIC study. European Journal of Neurology 2014;21:127‐8.
-
- Miller A, Wolinsky J, Kappos L, Comi G, Freedman M, Olsson T, et al. TOPIC: efficacy and safety of once‐daily oral teriflunomide in patients with first clinical episode consistent with multiple sclerosis. Neurology. 2014; Vol. 82. - PubMed
-
- Miller A, Wolinsky J, Kappos L, Comi G, Freedman MS, Olsson T, et al. TOPIC main outcomes: efficacy and safety of once‐daily oral teriflunomide in patients with clinically isolated syndrome. Multiple Sclerosis 2013;19:25‐6.
-
- Miller AE, Wolinsky JS, Kappos L, Comi G, Freedman MS, Olsson TP, et al. Oral teriflunomide for patients with a first clinical episode suggestive of multiple sclerosis (TOPIC): a randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet Neurology 2014;13(10):977‐86. - PubMed
-
- Turner B, Bauer D, Benamor M, Truffinet P, Miller A. Teriflunomide in early stage MS: results from TOPIC. Journal of Neurology, Neurosurgery and Psychiatry 2014;85:A40.
O'Connor 2006 {published data only}
-
- Jumah MA, Li DB, Yamout B, Truffinet P, Dukovic D, O'Connor PW. Long‐term clinical and magnetic resonance imaging outcomes from patients treated with teriflunomide: results from a phase 2 extension study. Multiple Sclerosis and Related Disorders 2014;3:755‐56.
-
- Li D, O'Connor P, Confavreux C, Truffinet P, Wang D, Traboulsee A. Efficacy of teriflunomide in relapsing multiple sclerosis: phase Ⅱ extension study with 8‐year follow‐up. Multiple Sclerosis 2011;17:S183‐4.
-
- Li DBK, Traboulsee AL, Truffinet P, Dukovic D, O'Connor PW. Long‐term MRI outcomes from patients treated with teriflunomide: results from a phase 2 extension study. Multiple Sclerosis 2014;20:102‐3.
Additional references
Bruneau 1998
Cella 1996
-
- Cella DF, Dineen K, Arnason B, Reder A, Webster KA, Karabatsos G, et al. Validation of the functional assessment of multiple sclerosis quality of life instrument. Neurology 1996;47(1):129‐39. - PubMed
Cherwinski 1995
-
- Cherwinski HM, McCarley D, Schatzman R, Devens B, Ransom JT. The immunosuppressant leflunomide inhibits lymphocyte progression through cell cycle by a novel mechanism. Journal of Pharmacology and Experimental Therapeutics 1995;272(1):460‐8. - PubMed
Confavreux 2006
-
- Confavreux C, Vukusic S. Natural history of multiple sclerosis: a unifying concept. Brain 2006;129(Pt 3):606‐16. - PubMed
Deage 1998
-
- Deage V, Burger D, Dayer JM. Exposure of T lymphocytes to leflunomide but not to dexamethasone favors the production by monocytic cells of interleukin‐1 receptor antagonist and the tissue‐inhibitor of metalloproteinases‐1 over that of interleukin‐1beta and metalloproteinases. European Cytokine Network 1998;9(4):663‐8. - PubMed
DerSimonian 1986
-
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. - PubMed
Fischer 1999
-
- Fischer JS, LaRocca NG, Miller DM, Ritvo PG, Andrews H, Paty D. Recent developments in the assessment of quality of life in multiple sclerosis (MS). Multiple Sclerosis 1999;5(4):251‐9. - PubMed
Greene 1995
-
- Greene S, Watanabe K, Braatz‐Trulson J, Lou L. Inhibition of dihydroorotate dehydrogenase by the immunosuppressive agent leflunomide. Biochemical Pharmacology 1995;50(6):861‐7. - PubMed
Greenland 1985
-
- Greenland S, Robins JM. Estimation of a common effect parameter from sparse follow‐up data. Biometrics 1985;41(1):55‐68. - PubMed
Hadjigeorgiou 2013
-
- Hadjigeorgiou GM, Doxani C, Miligkos M, Ziakas P, Bakalos G, Papadimitriou D, et al. A network meta‐analysis of randomized controlled trials for comparing the effectiveness and safety profile of treatments with marketing authorization for relapsing multiple sclerosis. Journal of Clinical Pharmacy and Therapeutics 2013;38(6):433‐9. - PubMed
Hamilton 1999
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Karampampa 2012
-
- Karampampa K, Gustavsson A, Miltenburger C, Kindundu CM, Selchen DH. Treatment experience, burden, and unmet needs (TRIBUNE) in multiple sclerosis: the costs and utilities of MS patients in Canada. Journal of Population Therapeutics and Clinical Pharmacology 2012;19(1):11‐25. - PubMed
Korn 2004
-
- Korn T, Magnus T, Toyka K, Jung S. Modulation of effector cell functions in experimental autoimmune encephalomyelitis by leflunomide ‐ mechanisms independent of pyrimidine depletion. Journal of Leukocyte Biology 2004;76:950‐60. - PubMed
Kurtzke 1983
-
- Kurtzke JF. Rating neurological impairment in multiple sclerosis: an Expanded Disability Status Scale (EDSS). Neurology 1983;33:1444‐52. - PubMed
Lublin 1996
-
- Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology 1996;46:907‐11. - PubMed
Lublin 2014a
Lublin 2014b
-
- Lublin FD. New multiple sclerosis phenotypic classification. European Neurology 2014;72 Suppl:1‐5. - PubMed
Manna 1999
-
- Manna SK, Aggarwal BB. Immunosuppressive leflunomide metabolite (A77 1726) blocks TNF‐dependent nuclear factor‐kappa B activation and gene expression. Journal of Immunology 1999;162(4):2095‐102. - PubMed
Mantel 1959
-
- Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. Journal of the National Cancer Institute 1959;22(4):719‐48. - PubMed
McDonald 2001
-
- McDonald WI, Compston A, Edan G, Goodkin D, Hartung HP, Lublin FD, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Annals of Neurology 2001;50(1):121‐7. - PubMed
Merrill 2009
-
- Merrill JE, Hanak S, Pu SF, Liang J, Dang C, Iglesias‐Bregna D, et al. Teriflunomide reduces behavioral, electrophysiological, and histopathological deficits in the Dark Agouti rat model of experimental autoimmune encephalomyelitis. Journal of Neurology 2009;256(1):89‐103. - PubMed
O'Connell 2014
-
- O'Connell K, Kelly SB, Fogarty E, Duggan M, Buckley L, Hutchinson M, et al. Economic costs associated with an MS relapse. Multiple Sclerosis and Related Disorders 2014;3(6):678‐83. - PubMed
Oleen‐Burkey 2012
-
- Oleen‐Burkey M, Castelli‐Haley J, Lage MJ, Johnson KP. Burden of a multiple sclerosis relapse: the patients perspective. Patient 2012;5(1):57‐69. - PubMed
Parisé 2013
-
- Parisé H, Laliberté F, Lefebvre P, Duh MS, Kim E, Agashivala N, et al. Direct and indirect cost burden associated with multiple sclerosis relapses: excess costs of persons with MS and their spouse caregivers. Journal of the Neurological Sciences 2013;330(1‐2):71‐7. - PubMed
Polman 2005
-
- Polman CH, Reingold SC, Edan G, Filippi M, Hartung HP, Kappos L, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the "McDonald Criteria". Annals of Neurology 2005;58(6):840‐6. - PubMed
Polman 2011
Poser 1983
-
- Poser CM, Paty DW, Scheinberg L, McDonald WI, Davis FA, Ebers GC, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Annals of Neurology 1983;13(3):227‐31. - PubMed
RevMan 2015 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2015.
Siemasko 1998
-
- Siemasko K, Chong AS, Jäck HM, Gong H, Williams JW, Finnegan A. Inhibition of JAK3 and STAT6 tyrosine phosphorylation by the immunosuppressive drug leflunomide leads to a block in IgG1 production. Journal of Immunology 1998;160(4):1581‐8. - PubMed
Tallantyre 2008
-
- Tallantyre E, Evangelou N, Constantinescu CS. Spotlight on teriflunomide. International MS Journal 2008;15(2):62‐8. - PubMed
Tramacere 2015
Tullman 2004
-
- Tullman MJ, Oshinsky RJ, Lublin FD, Cutter GR. Clinical characteristics of progressive relapsing multiple sclerosis. Multiple Sclerosis 2004;10(4):451‐4. - PubMed
Vickrey 1995
-
- Vickrey BG, Hays RD, Harooni R, Myers LW, Ellison GW. A health‐related quality of life measure for multiple sclerosis. Quality of Life Research 1995;4(3):187‐206. - PubMed
Ware 1992
-
- Ware JE Jr, Sherbourne CD. The MOS 36‐item short‐form health survey (SF‐36). I. Conceptual framework and item selection. Medical Care 1992;30(6):473‐83. - PubMed
Xu 1995
-
- Xu X, Williams JW, Bremer EG, Finnegan A, Chong AS. Inhibition of protein tyrosine phosphorylation in T cells by a novel immunosuppressive agent, leflunomide. Journal of Biological Chemistry 1995;270(21):12398‐403. - PubMed
Xu 1996
-
- Xu X, Williams JW, Gong H, Finnegan A, Chong AS. Two activities of the immunosuppressive metabolite of leflunomide, A77 1726. Inhibition of pyrimidine nucleotide synthesis and protein tyrosine phosphorylation. Biochemical Pharmacology 1996;52(4):527‐34. - PubMed
Zagmutt 2015
-
- Zagmutt FJ, Carroll CA. Meta‐analysis of adverse events in recent randomized clinical trials for dimethyl fumarate, glatiramer acetate and teriflunomide for the treatment of relapsing forms of multiple sclerosis. International Journal of Neuroscience 2015;125(11):798‐807. - PubMed
Zeyda 2005
-
- Zeyda M, Poglitsch M, Geyeregger R, Smolen JS, Zlabinger GJ, Hörl WH, et al. Disruption of the interaction of T cells with antigen‐presenting cells by the active leflunomide metabolite teriflunomide: involvement of impaired integrin activation and immunologic synapse formation. Arthritis and Rheumatism 2005;52(9):2730‐9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials