Naturally Acquired Antibody Responses to Plasmodium vivax and Plasmodium falciparum Merozoite Surface Protein 1 (MSP1) C-Terminal 19 kDa Domains in an Area of Unstable Malaria Transmission in Southeast Asia
- PMID: 26999435
- PMCID: PMC4801383
- DOI: 10.1371/journal.pone.0151900
Naturally Acquired Antibody Responses to Plasmodium vivax and Plasmodium falciparum Merozoite Surface Protein 1 (MSP1) C-Terminal 19 kDa Domains in an Area of Unstable Malaria Transmission in Southeast Asia
Abstract
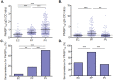
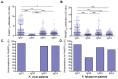
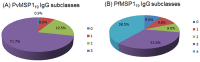
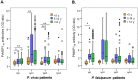
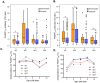
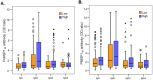
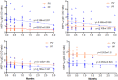
Understanding naturally acquired immunity to infections caused by Plasmodia in different malaria endemicity settings is needed for better vaccine designs and for exploring antibody responses as a proxy marker of malaria transmission intensity. This study investigated the sero-epidemiology of malaria along the international border between China and Myanmar, where malaria elimination action plans are in place. This study recruited 233 P. vivax and 156 P. falciparum infected subjects with acute malaria at the malaria clinics and hospitals. In addition, 93 and 67 healthy individuals from the same endemic region or from non-endemic region, respectively, were used as controls. Acute malaria infections were identified by microscopy. Anti-recombinant PfMSP119 and PvMSP119 antibody levels were measured by ELISA. Antibody responses to respective MSP119 were detected in 50.9% and 78.2% patients with acute P. vivax and P. falciparum infections, respectively. There were cross-reacting antibodies in Plasmodium patients against these two recombinant proteins, though we could not exclude the possibility of submicroscopic mixed-species infections. IgG1, IgG3 and IgG4 were the major subclasses. Interestingly, 43.2% of the healthy endemic population also had antibodies against PfMSP119, whereas only 3.9% of this population had antibodies against PvMSP119. Higher antibody levels were correlated with age and parasite density, but not with season, gender or malaria history. Both total IgG and individual IgG subclasses underwent substantial declines during the convalescent period in three months. This study demonstrated that individuals in a hypoendemic area with coexistence of P. vivax and P. falciparum can mount rapid antibody responses against both PfMSP119 and PvMSP119. The significantly higher proportion of responders to PfMSP119 in the healthy endemic population indicates higher prevalence of P. falciparum in the recent past. Specific antibodies against PvMSP119 could serve as a marker of recent exposure to P. vivax in epidemiological studies.
Conflict of interest statement
Figures
Similar articles
-
Antibody response to the N and C-terminal regions of the Plasmodium vivax Merozoite Surface Protein 1 in individuals living in an area of exclusive transmission of P. vivax malaria in the north of Brazil.Acta Trop. 1999 Jan 15;72(1):13-24. doi: 10.1016/s0001-706x(98)00078-3. Acta Trop. 1999. PMID: 9924957
-
Specificity of the IgG antibody response to Plasmodium falciparum, Plasmodium vivax, Plasmodium malariae, and Plasmodium ovale MSP119 subunit proteins in multiplexed serologic assays.Malar J. 2018 Nov 9;17(1):417. doi: 10.1186/s12936-018-2566-0. Malar J. 2018. PMID: 30413163 Free PMC article.
-
Comparative analysis of the profiles of IgG subclass-specific responses to Plasmodium falciparum apical membrane antigen-1 and merozoite surface protein-1 in naturally exposed individuals living in malaria hypoendemic settings, Iran.Malar J. 2015 Feb 5;14:58. doi: 10.1186/s12936-015-0547-0. Malar J. 2015. PMID: 25652589 Free PMC article.
-
Immunity against sexual stage Plasmodium falciparum and Plasmodium vivax parasites.Immunol Rev. 2020 Jan;293(1):190-215. doi: 10.1111/imr.12828. Epub 2019 Dec 16. Immunol Rev. 2020. PMID: 31840844 Free PMC article. Review.
-
The carboxy-terminus of merozoite surface protein 1: structure, specific antibodies and immunity to malaria.Parasitology. 2009 Oct;136(12):1445-56. doi: 10.1017/S0031182009990515. Epub 2009 Jul 23. Parasitology. 2009. PMID: 19627632 Review.
Cited by
-
Mosquito Bite-Induced Controlled Human Malaria Infection with Plasmodium vivax or P. falciparum Generates Immune Responses to Homologous and Heterologous Preerythrocytic and Erythrocytic Antigens.Infect Immun. 2019 Feb 21;87(3):e00541-18. doi: 10.1128/IAI.00541-18. Print 2019 Mar. Infect Immun. 2019. PMID: 30559218 Free PMC article.
-
Plasmodium vivax spleen-dependent genes encode antigens associated with cytoadhesion and clinical protection.Proc Natl Acad Sci U S A. 2020 Jun 9;117(23):13056-13065. doi: 10.1073/pnas.1920596117. Epub 2020 May 21. Proc Natl Acad Sci U S A. 2020. PMID: 32439708 Free PMC article.
-
Alternative Invasion Mechanisms and Host Immune Response to Plasmodium vivax Malaria: Trends and Future Directions.Microorganisms. 2020 Dec 23;9(1):15. doi: 10.3390/microorganisms9010015. Microorganisms. 2020. PMID: 33374596 Free PMC article. Review.
-
IgG subclass responses to excreted-secreted antigens of Plasmodium falciparum in a low-transmission malaria area of the Peruvian Amazon.Malar J. 2018 Sep 11;17(1):328. doi: 10.1186/s12936-018-2471-6. Malar J. 2018. PMID: 30200987 Free PMC article.
-
Genetic diversity of merozoite surface protein-1 C-terminal 42 kDa of Plasmodium falciparum (PfMSP-142) may be greater than previously known in global isolates.Parasit Vectors. 2018 Aug 6;11(1):455. doi: 10.1186/s13071-018-3027-x. Parasit Vectors. 2018. PMID: 30081943 Free PMC article.
References
-
- WHO (2014) World Malaria Report 2014.
-
- al-Yaman F, Genton B, Kramer KJ, Chang SP, Hui GS, et al. (1996) Assessment of the role of naturally acquired antibody levels to Plasmodium falciparum merozoite surface protein-1 in protecting Papua New Guinean children from malaria morbidity. Am J Trop Med Hyg 54: 443–448. - PubMed
-
- Branch OH, Udhayakumar V, Hightower AW, Oloo AJ, Hawley WA, et al. (1998) A longitudinal investigation of IgG and IgM antibody responses to the merozoite surface protein-1 19-kiloDalton domain of Plasmodium falciparum in pregnant women and infants: associations with febrile illness, parasitemia, and anemia. Am J Trop Med Hyg 58: 211–219. - PubMed
-
- Riley EM, Allen SJ, Wheeler JG, Blackman MJ, Bennett S, et al. (1992) Naturally acquired cellular and humoral immune responses to the major merozoite surface antigen (PfMSP1) of Plasmodium falciparum are associated with reduced malaria morbidity. Parasite Immunol 14: 321–337. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

