Elevated expression of NEU1 sialidase in idiopathic pulmonary fibrosis provokes pulmonary collagen deposition, lymphocytosis, and fibrosis
- PMID: 26993524
- PMCID: PMC4896097
- DOI: 10.1152/ajplung.00346.2015
Elevated expression of NEU1 sialidase in idiopathic pulmonary fibrosis provokes pulmonary collagen deposition, lymphocytosis, and fibrosis
Abstract
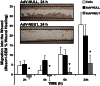
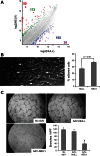
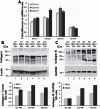
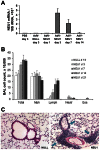
Idiopathic pulmonary fibrosis (IPF) poses challenges to understanding its underlying cellular and molecular mechanisms and the development of better therapies. Previous studies suggest a pathophysiological role for neuraminidase 1 (NEU1), an enzyme that removes terminal sialic acid from glycoproteins. We observed increased NEU1 expression in epithelial and endothelial cells, as well as fibroblasts, in the lungs of patients with IPF compared with healthy control lungs. Recombinant adenovirus-mediated gene delivery of NEU1 to cultured primary human cells elicited profound changes in cellular phenotypes. Small airway epithelial cell migration was impaired in wounding assays, whereas, in pulmonary microvascular endothelial cells, NEU1 overexpression strongly impacted global gene expression, increased T cell adhesion to endothelial monolayers, and disrupted endothelial capillary-like tube formation. NEU1 overexpression in fibroblasts provoked increased levels of collagen types I and III, substantial changes in global gene expression, and accelerated degradation of matrix metalloproteinase-14. Intratracheal instillation of NEU1 encoding, but not control adenovirus, induced lymphocyte accumulation in bronchoalveolar lavage samples and lung tissues and elevations of pulmonary transforming growth factor-β and collagen. The lymphocytes were predominantly T cells, with CD8(+) cells exceeding CD4(+) cells by nearly twofold. These combined data indicate that elevated NEU1 expression alters functional activities of distinct lung cell types in vitro and recapitulates lymphocytic infiltration and collagen accumulation in vivo, consistent with mechanisms implicated in lung fibrosis.
Keywords: fibroblasts; fibrosis; inflammation.
Figures




Similar articles
-
Therapeutic Effect of Neuraminidase-1-Selective Inhibition in Mouse Models of Bleomycin-Induced Pulmonary Inflammation and Fibrosis.J Pharmacol Exp Ther. 2021 Jan;376(1):136-146. doi: 10.1124/jpet.120.000223. Epub 2020 Nov 2. J Pharmacol Exp Ther. 2021. PMID: 33139318 Free PMC article.
-
The NEU1-selective sialidase inhibitor, C9-butyl-amide-DANA, blocks sialidase activity and NEU1-mediated bioactivities in human lung in vitro and murine lung in vivo.Glycobiology. 2016 Aug;26(8):834-49. doi: 10.1093/glycob/cww060. Epub 2016 May 25. Glycobiology. 2016. PMID: 27226251 Free PMC article.
-
NEU1 sialidase regulates the sialylation state of CD31 and disrupts CD31-driven capillary-like tube formation in human lung microvascular endothelia.J Biol Chem. 2014 Mar 28;289(13):9121-35. doi: 10.1074/jbc.M114.555888. Epub 2014 Feb 18. J Biol Chem. 2014. PMID: 24550400 Free PMC article.
-
Role of matrix metalloproteinases in the pathogenesis of idiopathic pulmonary fibrosis.Respir Res. 2016 Mar 4;17:23. doi: 10.1186/s12931-016-0343-6. Respir Res. 2016. PMID: 26944412 Free PMC article. Review.
-
Neuraminidase 1 and its Inhibitors from Chinese Herbal Medicines: An Emerging Role for Cardiovascular Diseases.Am J Chin Med. 2021;49(4):843-862. doi: 10.1142/S0192415X21500403. Epub 2021 Apr 6. Am J Chin Med. 2021. PMID: 33827385 Review.
Cited by
-
Inhibitors of Human Neuraminidase Enzymes Block Transmigration in vitro.Front Mol Biosci. 2022 Feb 25;9:835757. doi: 10.3389/fmolb.2022.835757. eCollection 2022. Front Mol Biosci. 2022. PMID: 35281276 Free PMC article.
-
Neuraminidase 1-mediated desialylation of the mucin 1 ectodomain releases a decoy receptor that protects against Pseudomonas aeruginosa lung infection.J Biol Chem. 2019 Jan 11;294(2):662-678. doi: 10.1074/jbc.RA118.006022. Epub 2018 Nov 14. J Biol Chem. 2019. PMID: 30429216 Free PMC article.
-
The role of sialidase Neu1 in respiratory diseases.Respir Res. 2024 Mar 19;25(1):134. doi: 10.1186/s12931-024-02763-9. Respir Res. 2024. PMID: 38500102 Free PMC article. Review.
-
Reduced Sialylation and Bioactivity of the Antifibrotic Protein Serum Amyloid P in the Sera of Patients with Idiopathic Pulmonary Fibrosis.Immunohorizons. 2020 Jun 23;4(6):352-362. doi: 10.4049/immunohorizons.2000043. Immunohorizons. 2020. PMID: 32576593 Free PMC article.
-
Therapeutic Effect of Neuraminidase-1-Selective Inhibition in Mouse Models of Bleomycin-Induced Pulmonary Inflammation and Fibrosis.J Pharmacol Exp Ther. 2021 Jan;376(1):136-146. doi: 10.1124/jpet.120.000223. Epub 2020 Nov 2. J Pharmacol Exp Ther. 2021. PMID: 33139318 Free PMC article.
References
-
- Abdulkhalek S, Szewczuk MR. Neu1 sialidase and matrix metalloproteinase-9 cross-talk regulates nucleic acid-induced endosomal TOLL-like receptor-7 and -9 activation, cellular signaling and pro-inflammatory responses. Cell Signal 25: 2093–2105, 2013. - PubMed
-
- Abe M, Harpel JG, Metz CN, Nunes I, Loskutoff DJ, Rifkin DB. An assay for transforming growth factor-beta using cells transfected with a plasminogen activator inhibitor-1 promoter-luciferase construct. Anal Biochem 216: 276–284, 1994. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

