A Pharmacogenetic Discovery: Cystamine Protects Against Haloperidol-Induced Toxicity and Ischemic Brain Injury
- PMID: 26993135
- PMCID: PMC4858802
- DOI: 10.1534/genetics.115.184648
A Pharmacogenetic Discovery: Cystamine Protects Against Haloperidol-Induced Toxicity and Ischemic Brain Injury
Abstract
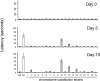
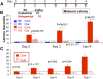
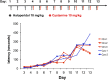
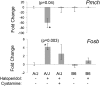
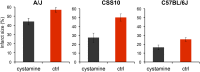
Haloperidol is an effective antipsychotic agent, but it causes Parkinsonian-like extrapyramidal symptoms in the majority of treated subjects. To address this treatment-limiting toxicity, we analyzed a murine genetic model of haloperidol-induced toxicity (HIT). Analysis of a panel of consomic strains indicated that a genetic factor on chromosome 10 had a significant effect on susceptibility to HIT. We analyzed a whole-genome SNP database to identify allelic variants that were uniquely present on chromosome 10 in the strain that was previously shown to exhibit the highest level of susceptibility to HIT. This analysis implicated allelic variation within pantetheinase genes (Vnn1 and Vnn3), which we propose impaired the biosynthesis of cysteamine, could affect susceptibility to HIT. We demonstrate that administration of cystamine, which is rapidly metabolized to cysteamine, could completely prevent HIT in the murine model. Many of the haloperidol-induced gene expression changes in the striatum of the susceptible strain were reversed by cystamine coadministration. Since cystamine administration has previously been shown to have other neuroprotective actions, we investigated whether cystamine administration could have a broader neuroprotective effect. Cystamine administration caused a 23% reduction in infarct volume after experimentally induced cerebral ischemia. Characterization of this novel pharmacogenetic factor for HIT has identified a new approach for preventing the treatment-limiting toxicity of an antipsychotic agent, which could also be used to reduce the extent of brain damage after stroke.
Keywords: haloperidol toxicity; pharmacogenetics.
Copyright © 2016 by the Genetics Society of America.
Figures
Similar articles
-
The role of Abcb5 alleles in susceptibility to haloperidol-induced toxicity in mice and humans.PLoS Med. 2015 Feb 3;12(2):e1001782. doi: 10.1371/journal.pmed.1001782. eCollection 2015 Feb. PLoS Med. 2015. PMID: 25647612 Free PMC article.
-
Complex genetic control of susceptibility to malaria: positional cloning of the Char9 locus.J Exp Med. 2007 Mar 19;204(3):511-24. doi: 10.1084/jem.20061252. Epub 2007 Feb 20. J Exp Med. 2007. PMID: 17312006 Free PMC article.
-
Vanin-1-/- mice exhibit a glutathione-mediated tissue resistance to oxidative stress.Mol Cell Biol. 2004 Aug;24(16):7214-24. doi: 10.1128/MCB.24.16.7214-7224.2004. Mol Cell Biol. 2004. PMID: 15282320 Free PMC article.
-
Influence of Vanin-1 and Catalytic Products in Liver During Normal and Oxidative Stress Conditions.Curr Med Chem. 2015;22(20):2407-16. doi: 10.2174/092986732220150722124307. Curr Med Chem. 2015. PMID: 26549544 Review.
-
Potential of cystamine and cysteamine in the treatment of neurodegenerative diseases.Prog Neuropsychopharmacol Biol Psychiatry. 2011 Mar 30;35(2):380-9. doi: 10.1016/j.pnpbp.2010.11.023. Epub 2010 Nov 24. Prog Neuropsychopharmacol Biol Psychiatry. 2011. PMID: 21111020 Review.
Cited by
-
What Have We Learned (or Expect to) From Analysis of Murine Genetic Models Related to Substance Use Disorders?Front Psychiatry. 2022 Jan 12;12:793961. doi: 10.3389/fpsyt.2021.793961. eCollection 2021. Front Psychiatry. 2022. PMID: 35095607 Free PMC article. Review.
-
An automated multi-modal graph-based pipeline for mouse genetic discovery.Bioinformatics. 2022 Jun 27;38(13):3385-3394. doi: 10.1093/bioinformatics/btac356. Bioinformatics. 2022. PMID: 35608290 Free PMC article.
-
The Effect of Population Structure on Murine Genome-Wide Association Studies.Front Genet. 2021 Sep 13;12:745361. doi: 10.3389/fgene.2021.745361. eCollection 2021. Front Genet. 2021. PMID: 34589118 Free PMC article.
-
Therapeutic Applications of Cysteamine and Cystamine in Neurodegenerative and Neuropsychiatric Diseases.Front Neurol. 2019 Dec 12;10:1315. doi: 10.3389/fneur.2019.01315. eCollection 2019. Front Neurol. 2019. PMID: 31920936 Free PMC article. Review.
References
-
- Benjamini Y., Hochberg Y., 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. B 57: 289–300.
-
- Beresford R., Ward A., 1987. Haloperidol decanoate. A preliminary review of its pharmacodynamic and pharmacokinetic properties and therapeutic use in psychosis. Drugs 33: 31–49. - PubMed
-
- Bloomquist J., King E., Wright A., Mytilineou C., Kimura K., et al. , 1994. 1-Methyl-4-phenylpyridinium-like neurotoxicity of a pyridinium metabolite derived from haloperidol: cell culture and neurotransmitter uptake studies. J. Pharmacol. Exp. Ther. 270: 822–830. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

