Natural Genetic Variation Influences Protein Abundances in C. elegans Developmental Signalling Pathways
- PMID: 26985669
- PMCID: PMC4795773
- DOI: 10.1371/journal.pone.0149418
Natural Genetic Variation Influences Protein Abundances in C. elegans Developmental Signalling Pathways
Abstract
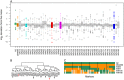
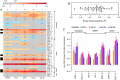
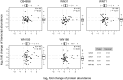
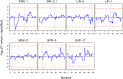
Complex traits, including common disease-related traits, are affected by many different genes that function in multiple pathways and networks. The apoptosis, MAPK, Notch, and Wnt signalling pathways play important roles in development and disease progression. At the moment we have a poor understanding of how allelic variation affects gene expression in these pathways at the level of translation. Here we report the effect of natural genetic variation on transcript and protein abundance involved in developmental signalling pathways in Caenorhabditis elegans. We used selected reaction monitoring to analyse proteins from the abovementioned four pathways in a set of recombinant inbred lines (RILs) generated from the wild-type strains N2 (Bristol) and CB4856 (Hawaii) to enable quantitative trait locus (QTL) mapping. About half of the cases from the 44 genes tested showed a statistically significant change in protein abundance between various strains, most of these were however very weak (below 1.3-fold change). We detected a distant QTL on the left arm of chromosome II that affected protein abundance of the phosphatidylserine receptor protein PSR-1, and two separate QTLs that influenced embryonic and ionizing radiation-induced apoptosis on chromosome IV. Our results demonstrate that natural variation in C. elegans is sufficient to cause significant changes in signalling pathways both at the gene expression (transcript and protein abundance) and phenotypic levels.
Conflict of interest statement
Figures


Similar articles
-
Punctuated Loci on Chromosome IV Determine Natural Variation in Orsay Virus Susceptibility of Caenorhabditis elegans Strains Bristol N2 and Hawaiian CB4856.J Virol. 2021 May 24;95(12):e02430-20. doi: 10.1128/JVI.02430-20. Print 2021 May 24. J Virol. 2021. PMID: 33827942 Free PMC article.
-
A multi-parent recombinant inbred line population of C. elegans allows identification of novel QTLs for complex life history traits.BMC Biol. 2019 Mar 12;17(1):24. doi: 10.1186/s12915-019-0642-8. BMC Biol. 2019. PMID: 30866929 Free PMC article.
-
Linkage mapping reveals loci that underlie differences in Caenorhabditis elegans growth.G3 (Bethesda). 2022 Sep 30;12(10):jkac207. doi: 10.1093/g3journal/jkac207. G3 (Bethesda). 2022. PMID: 35961034 Free PMC article.
-
Natural genetic variation as a tool for discovery in Caenorhabditis nematodes.Genetics. 2022 Jan 4;220(1):iyab156. doi: 10.1093/genetics/iyab156. Genetics. 2022. PMID: 35134197 Free PMC article. Review.
-
Regulation of aging and innate immunity in C. elegans.Aging Cell. 2004 Aug;3(4):185-93. doi: 10.1111/j.1474-9728.2004.00108.x. Aging Cell. 2004. PMID: 15268752 Review.
Cited by
-
Proteome-wide systems genetics identifies UFMylation as a regulator of skeletal muscle function.Elife. 2022 Dec 6;11:e82951. doi: 10.7554/eLife.82951. Elife. 2022. PMID: 36472367 Free PMC article.
-
Illuminating Biological Interactions with in Vivo Protein Footprinting.Anal Chem. 2019 May 21;91(10):6577-6584. doi: 10.1021/acs.analchem.9b00244. Epub 2019 May 7. Anal Chem. 2019. PMID: 31025855 Free PMC article.
-
WormQTL2: an interactive platform for systems genetics in Caenorhabditis elegans.Database (Oxford). 2020 Jan 1;2020:baz149. doi: 10.1093/database/baz149. Database (Oxford). 2020. PMID: 31960906 Free PMC article.
-
From QTL to gene: C. elegans facilitates discoveries of the genetic mechanisms underlying natural variation.Trends Genet. 2021 Oct;37(10):933-947. doi: 10.1016/j.tig.2021.06.005. Epub 2021 Jul 3. Trends Genet. 2021. PMID: 34229867 Free PMC article. Review.
-
The Gene scb-1 Underlies Variation in Caenorhabditis elegans Chemotherapeutic Responses.G3 (Bethesda). 2020 Jul 7;10(7):2353-2364. doi: 10.1534/g3.120.401310. G3 (Bethesda). 2020. PMID: 32385045 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

