Discovery of G Protein-Biased EP2 Receptor Agonists
- PMID: 26985320
- PMCID: PMC4789666
- DOI: 10.1021/acsmedchemlett.5b00455
Discovery of G Protein-Biased EP2 Receptor Agonists
Abstract
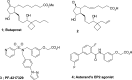
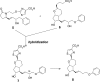
To identify G protein-biased and highly subtype-selective EP2 receptor agonists, a series of bicyclic prostaglandin analogues were designed and synthesized. Structural hybridization of EP2/4 dual agonist 5 and prostacyclin analogue 6, followed by simplification of the ω chain enabled us to discover novel EP2 agonists with a unique prostacyclin-like scaffold. Further optimization of the ω chain was performed to improve EP2 agonist activity and subtype selectivity. Phenoxy derivative 18a showed potent agonist activity and excellent subtype selectivity. Furthermore, a series of compounds were identified as G protein-biased EP2 receptor agonists. These are the first examples of biased ligands of prostanoid receptors.
Keywords: EP2; Prostaglandin; agonist; biased ligand; structure−functional selectivity relationship.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
Structural optimization and structure-functional selectivity relationship studies of G protein-biased EP2 receptor agonists.Bioorg Med Chem Lett. 2016 May 15;26(10):2446-2449. doi: 10.1016/j.bmcl.2016.03.110. Epub 2016 Mar 31. Bioorg Med Chem Lett. 2016. PMID: 27055938
-
Characterization of the prostanoid receptor(s) on human blood monocytes at which prostaglandin E2 inhibits lipopolysaccharide-induced tumour necrosis factor-alpha generation.Br J Pharmacol. 1997 Sep;122(1):149-57. doi: 10.1038/sj.bjp.0701360. Br J Pharmacol. 1997. PMID: 9298541 Free PMC article.
-
Discovery of novel prostaglandin analogs as potent and selective EP2/EP4 dual agonists.Bioorg Med Chem. 2012 Apr 1;20(7):2235-51. doi: 10.1016/j.bmc.2012.02.018. Epub 2012 Feb 16. Bioorg Med Chem. 2012. PMID: 22386979
-
The affinity, intrinsic activity and selectivity of a structurally novel EP2 receptor agonist at human prostanoid receptors.Br J Pharmacol. 2019 Mar;176(5):687-698. doi: 10.1111/bph.14525. Epub 2019 Jan 4. Br J Pharmacol. 2019. PMID: 30341781 Free PMC article.
-
[Cooperation of two subtypes of PGE2 receptor, Gi coupled EP3 and Gs coupled EP2 or EP4 subtype].Yakugaku Zasshi. 2003 Oct;123(10):837-43. doi: 10.1248/yakushi.123.837. Yakugaku Zasshi. 2003. PMID: 14577329 Review. Japanese.
Cited by
-
BiasNet: A Model to Predict Ligand Bias Toward GPCR Signaling.J Chem Inf Model. 2021 Sep 27;61(9):4190-4199. doi: 10.1021/acs.jcim.1c00317. Epub 2021 Aug 16. J Chem Inf Model. 2021. PMID: 34397210 Free PMC article.
-
Direct Cα-heteroarylation of structurally diverse ethers via a mild N-hydroxysuccinimide mediated cross-dehydrogenative coupling reaction.Chem Sci. 2017 May 1;8(5):4044-4050. doi: 10.1039/c6sc05697k. Epub 2017 Mar 24. Chem Sci. 2017. PMID: 30155212 Free PMC article.
-
EP2 inhibition restores myeloid metabolism and reverses cognitive decline.J Allergy Clin Immunol Glob. 2023 Feb 8;2(2):100082. doi: 10.1016/j.jacig.2023.100082. eCollection 2023 May. J Allergy Clin Immunol Glob. 2023. PMID: 37780795 Free PMC article.
-
Novel high-throughput myofibroblast assays identify agonists with therapeutic potential in pulmonary fibrosis that act via EP2 and EP4 receptors.PLoS One. 2018 Nov 28;13(11):e0207872. doi: 10.1371/journal.pone.0207872. eCollection 2018. PLoS One. 2018. PMID: 30485339 Free PMC article.
References
-
- Coleman R. A.; Kennedy I.; Humphrey P. P. A.; Bunce K.; Lumley P. In Comprehensive Medicinal Chemistry; Hansch C., Sammes P. G., Taylor J. B., Emmett J. C., Eds.; Pergamon: Oxford, 1990; Vol. 3, pp 643–714.
-
- Yoshida K.; Oida H.; Kobayashi T.; Maruyama T.; Tanaka M.; Katayama T.; Yamaguchi K.; Segi E.; Tsuboyama T.; Matsushita M.; Ito K.; Ito Y.; Sugimoto Y.; Ushikubi F.; Ohuchida S.; Kondo K.; Nakamura T.; Narumiya S. Stimulation of bone formation and prevention of bone loss by prostaglandin E EP4 receptor activation. Proc. Natl. Acad. Sci. U. S. A. 2002, 99, 4580–4585. 10.1073/pnas.062053399. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

