Extracranial injections of botulinum neurotoxin type A inhibit intracranial meningeal nociceptors' responses to stimulation of TRPV1 and TRPA1 channels: Are we getting closer to solving this puzzle?
- PMID: 26984967
- PMCID: PMC4959034
- DOI: 10.1177/0333102416636843
Extracranial injections of botulinum neurotoxin type A inhibit intracranial meningeal nociceptors' responses to stimulation of TRPV1 and TRPA1 channels: Are we getting closer to solving this puzzle?
Abstract
Background: Administration of onabotulinumtoxinA (BoNT-A) to peripheral tissues outside the calvaria reduces the number of days chronic migraine patients experience headache. Because the headache phase of a migraine attack, especially those preceded by aura, is thought to involve activation of meningeal nociceptors by endogenous stimuli such as changes in intracranial pressure (i.e. mechanical) or chemical irritants that appear in the meninges as a result of a yet-to-be-discovered sequence of molecular/cellular events triggered by the aura, we sought to determine whether extracranial injections of BoNT-A alter the chemosensitivity of meningeal nociceptors to stimulation of their intracranial receptive fields.
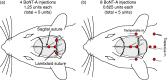
Material and methods: Using electrophysiological techniques, we identified 161 C- and 135 Aδ-meningeal nociceptors in rats and determined their mechanical response threshold and responsiveness to chemical stimulation of their dural receptive fields with TRPV1 and TRPA1 agonists seven days after BoNT-A administration to different extracranial sites. Two paradigms were compared: distribution of 5 U BoNT-A to the lambdoid and sagittal sutures alone, and 1.25 U to the sutures and 3.75 U to the temporalis and trapezius muscles.
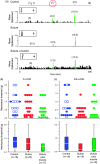
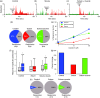
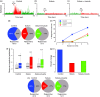
Results: Seven days after it was administered to tissues outside the calvaria, BoNT-A inhibited responses of C-type meningeal nociceptors to stimulation of their intracranial dural receptive fields with the TRPV1 agonist capsaicin and the TRPA1 agonist mustard oil. BoNT-A inhibition of responses to capsaicin was more effective when the entire dose was injected along the suture lines than when it was injected into muscles and sutures. As in our previous study, BoNT-A had no effect on non-noxious mechanosensitivity of C-fibers or on responsiveness of Aδ-fibers to mechanical and chemical stimulation.
Discussion: This study demonstrates that extracranial administration of BoNT-A suppresses meningeal nociceptors' responses to stimulation of their intracranial dural receptive fields with capsaicin and mustard oil. The findings suggest that surface expression of TRPV1 and TRPA1 channels in dural nerve endings of meningeal nociceptors is reduced seven days after extracranial administration of BoNT-A. In the context of chronic migraine, reduced sensitivity to molecules that activate meningeal nociceptors through the TRPV1 and TRPA1 channels can be important for BoNT-A's ability to act as a prophylactic.
Keywords: Headache; chronic migraine; migraine prophylaxis; pain; trigeminovascular.
© International Headache Society 2016.
Figures
Similar articles
-
Selective inhibition of meningeal nociceptors by botulinum neurotoxin type A: therapeutic implications for migraine and other pains.Cephalalgia. 2014 Oct;34(11):853-69. doi: 10.1177/0333102414527648. Epub 2014 Apr 2. Cephalalgia. 2014. PMID: 24694964 Free PMC article.
-
Exploring the effects of extracranial injections of botulinum toxin type A on prolonged intracranial meningeal nociceptors responses to cortical spreading depression in female rats.Cephalalgia. 2019 Oct;39(11):1358-1365. doi: 10.1177/0333102419873675. Epub 2019 Aug 31. Cephalalgia. 2019. PMID: 31475573 Free PMC article.
-
Activation of TRPV1 and TRPA1 leads to muscle nociception and mechanical hyperalgesia.Pain. 2009 Aug;144(3):270-277. doi: 10.1016/j.pain.2009.04.021. Epub 2009 May 22. Pain. 2009. PMID: 19464796 Free PMC article.
-
Extracranial origin of headache.Curr Opin Neurol. 2017 Jun;30(3):263-271. doi: 10.1097/WCO.0000000000000437. Curr Opin Neurol. 2017. PMID: 28248698 Free PMC article. Review.
-
Differential effects of TRPV channel block on polymodal activation of rat cutaneous nociceptors in vitro.Exp Brain Res. 2009 Jun;196(1):31-44. doi: 10.1007/s00221-009-1808-3. Epub 2009 Apr 30. Exp Brain Res. 2009. PMID: 19404626 Review.
Cited by
-
OnabotulinumtoxinA alters inflammatory gene expression and immune cells in chronic headache patients.Brain. 2022 Jul 29;145(7):2436-2449. doi: 10.1093/brain/awab461. Brain. 2022. PMID: 34932787 Free PMC article.
-
The pluripotential evolution and journey of Botox (onabotulinumtoxinA).Medicine (Baltimore). 2023 Jul 1;102(S1):e32373. doi: 10.1097/MD.0000000000032373. Medicine (Baltimore). 2023. PMID: 37499079 Free PMC article. Review.
-
Epigenetic Connections of the TRPA1 Ion Channel in Pain Transmission and Neurogenic Inflammation - a Therapeutic Perspective in Migraine?Mol Neurobiol. 2023 Oct;60(10):5578-5591. doi: 10.1007/s12035-023-03428-2. Epub 2023 Jun 16. Mol Neurobiol. 2023. PMID: 37326902 Free PMC article. Review.
-
Chronic Migraine and Medication Overuse Headache Worsening After OnabotulinumtoxinA Withdrawn Due to the Severe Acute Respiratory Syndrome-Coronavirus-2 Pandemic.Front Neurol. 2021 Apr 15;12:647995. doi: 10.3389/fneur.2021.647995. eCollection 2021. Front Neurol. 2021. PMID: 33935945 Free PMC article.
-
Novel insight into atogepant mechanisms of action in migraine prevention.Brain. 2024 Aug 1;147(8):2884-2896. doi: 10.1093/brain/awae062. Brain. 2024. PMID: 38411458 Free PMC article.
References
-
- Aurora SK, Dodick DW, Turkel CC, et al. OnabotulinumtoxinA for treatment of chronic migraine: Results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 1 trial. Cephalalgia 2010; 30: 793–803. - PubMed
-
- Diener HC, Dodick DW, Aurora SK, et al. OnabotulinumtoxinA for treatment of chronic migraine: Results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalalgia 2010; 30: 804–814. - PubMed
-
- Bolay H, Reuter U, Dunn AK, et al. Intrinsic brain activity triggers trigeminal meningeal afferents in a migraine model. Nat Med 2002; 8: 136–142. - PubMed
-
- Noseda R, Burstein R. Migraine pathophysiology: Anatomy of the trigeminovascular pathway and associated neurological symptoms, cortical spreading depression, sensitization, and modulation of pain. Pain 2013; 154(Suppl 1): S44–S53. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

