ERα propelled aberrant global DNA hypermethylation by activating the DNMT1 gene to enhance anticancer drug resistance in human breast cancer cells
- PMID: 26980709
- PMCID: PMC4991505
- DOI: 10.18632/oncotarget.8038
ERα propelled aberrant global DNA hypermethylation by activating the DNMT1 gene to enhance anticancer drug resistance in human breast cancer cells
Abstract
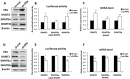
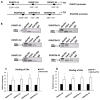
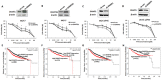
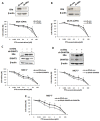
Drug-induced aberrant DNA methylation is the first identified epigenetic marker involved in chemotherapy resistance. Understanding how the aberrant DNA methylation is acquired would impact cancer treatment in theory and practice. In this study we systematically investigated whether and how ERα propelled aberrant global DNA hypermethylation in the context of breast cancer drug resistance. Our data demonstrated that anticancer drug paclitaxel (PTX) augmented ERα binding to the DNMT1 and DNMT3b promoters to activate DNMT1 and DNMT3b genes, enhancing the PTX resistance of breast cancer cells. In support of these observations, estrogen enhanced multi-drug resistance of breast cancer cells by up-regulation of DNMT1 and DNMT3b genes. Nevertheless, the aberrant global DNA hypermethylation was dominantly induced by ERα-activated-DNMT1, since DNMT1 over-expression significantly increased global DNA methylation and DNMT1 knockdown reversed the ERα-induced global DNA methylation. Altering DNMT3b expression had no detectable effect on global DNA methylation. Consistently, the expression level of DNMT1 was positively correlated with ERα in 78 breast cancer tissue samples shown by our immunohistochemistry (IHC) analysis and negatively correlated with relapse-free survival (RFS) and distance metastasis-free survival (DMFS) of ERα-positive breast cancer patients. This study provides a new perspective for understanding the mechanism underlying drug-resistance-facilitating aberrant DNA methylation in breast cancer and other estrogen dependent tumors.
Keywords: DNMT1; DNMT3b; ERα; breast cancer chemoresistance; global DNA hypermethylation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
ERα positively regulated DNMT1 expression by binding to the gene promoter region in human breast cancer MCF-7 cells.Biochem Biophys Res Commun. 2012 Oct 12;427(1):47-53. doi: 10.1016/j.bbrc.2012.08.144. Epub 2012 Sep 10. Biochem Biophys Res Commun. 2012. PMID: 22975348
-
Arsenic induces functional re-expression of estrogen receptor α by demethylation of DNA in estrogen receptor-negative human breast cancer.PLoS One. 2012;7(4):e35957. doi: 10.1371/journal.pone.0035957. Epub 2012 Apr 27. PLoS One. 2012. PMID: 22558281 Free PMC article.
-
Expression and clinicopathological significance of DNA methyltransferase 1, 3A and 3B in tamoxifen-treated breast cancer patients.Gene. 2019 Feb 15;685:24-31. doi: 10.1016/j.gene.2018.10.060. Epub 2018 Oct 23. Gene. 2019. PMID: 30359738
-
DNMT1: A key drug target in triple-negative breast cancer.Semin Cancer Biol. 2021 Jul;72:198-213. doi: 10.1016/j.semcancer.2020.05.010. Epub 2020 May 24. Semin Cancer Biol. 2021. PMID: 32461152 Review.
-
Crosstalk of methylation and tamoxifen in breast cancer (Review).Mol Med Rep. 2024 Oct;30(4):180. doi: 10.3892/mmr.2024.13304. Epub 2024 Aug 12. Mol Med Rep. 2024. PMID: 39129315 Free PMC article. Review.
Cited by
-
Interaction of WBP2 with ERα increases doxorubicin resistance of breast cancer cells by modulating MDR1 transcription.Br J Cancer. 2018 May 1;119(2):182-192. doi: 10.1038/s41416-018-0119-5. Br J Cancer. 2018. PMID: 29937544 Free PMC article.
-
PGK1-mediated cancer progression and drug resistance.Am J Cancer Res. 2019 Nov 1;9(11):2280-2302. eCollection 2019. Am J Cancer Res. 2019. PMID: 31815035 Free PMC article. Review.
-
PGK1 : An Essential Player in Modulating Tumor Metabolism.Methods Mol Biol. 2022;2343:57-70. doi: 10.1007/978-1-0716-1558-4_4. Methods Mol Biol. 2022. PMID: 34473315
-
Single-Cell Image-Based Analysis Reveals Chromatin Changes during the Acquisition of Tamoxifen Drug Resistance.Life (Basel). 2022 Mar 17;12(3):438. doi: 10.3390/life12030438. Life (Basel). 2022. PMID: 35330189 Free PMC article.
-
TFDP3 confers chemoresistance in minimal residual disease within childhood T-cell acute lymphoblastic leukemia.Oncotarget. 2017 Jan 3;8(1):1405-1415. doi: 10.18632/oncotarget.13630. Oncotarget. 2017. PMID: 27902457 Free PMC article.
References
-
- Yegnasubramanian S, Haffner MC, Zhang YG, Gurel B, Cornish TC, Wu ZJ, Irizarry RA, Morgan J, Hicks J, DeWeese TL, Isaacs WB, Bova GS, De Marzo AM, Nelson WG. DNA Hypomethylation Arises Later in Prostate Cancer Progression than CpG Island Hypermethylation and Contributes to Metastatic Tumor Heterogeneity. Cancer Research. 2008;68:8954–8967. - PMC - PubMed
-
- Lujambio A, Esteller M. How epigenetics can explain human metastasis A new role for microRNAs. Cell Cycle. 2009;8:377–382. - PubMed
-
- Nyce J. Drug-induced DNA hypermethylation and drug resistance in human tumors. Cancer Res. 1989;49:5829–5836. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

