The Basis of Oncoimmunology
- PMID: 26967289
- PMCID: PMC4788788
- DOI: 10.1016/j.cell.2016.01.049
The Basis of Oncoimmunology
Abstract
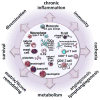
Cancer heterogeneity, a hallmark enabling clonal survival and therapy resistance, is shaped by active immune responses. Antigen-specific T cells can control cancer, as revealed clinically by immunotherapeutics such as adoptive T-cell transfer and checkpoint blockade. The host immune system is thus a powerful tool that, if better harnessed, could significantly enhance the efficacy of cytotoxic therapy and improve outcomes for cancer sufferers. To realize this vision, however, a number of research frontiers must be tackled. These include developing strategies for neutralizing tumor-promoting inflammation, broadening T-cell repertoires (via vaccination), and elucidating the mechanisms by which immune cells organize tumor microenvironments to regulate T-cell activity. Such efforts will pave the way for identifying new targets for combination therapies that overcome resistance to current treatments and promote long-term cancer control.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Generation of tumor-specific T-cell therapies.Blood Rev. 2006 Mar;20(2):61-9. doi: 10.1016/j.blre.2005.05.001. Epub 2005 Jun 22. Blood Rev. 2006. PMID: 15978709 Review.
-
Immunosuppressive mechanisms in cancer: consequences for the development of therapeutic vaccines.Vaccine. 2009 May 26;27(25-26):3398-400. doi: 10.1016/j.vaccine.2009.01.070. Epub 2009 Feb 5. Vaccine. 2009. PMID: 19200836
-
Adoptive T-cell therapy: a need for standard immune monitoring.Immunotherapy. 2015;7(5):513-33. doi: 10.2217/imt.15.23. Immunotherapy. 2015. PMID: 26065477 Review.
-
Cancer immunotherapy and breaking immune tolerance: new approaches to an old challenge.Cancer Res. 2015 Jan 1;75(1):5-10. doi: 10.1158/0008-5472.CAN-14-2538. Epub 2014 Dec 18. Cancer Res. 2015. PMID: 25524899 Free PMC article. Review.
-
A Primer for Oncoimmunology (Immunooncology).Toxicol Pathol. 2017 Jul;45(5):584-588. doi: 10.1177/0192623317713318. Epub 2017 Jun 21. Toxicol Pathol. 2017. PMID: 28635517 Review.
Cited by
-
Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors.Nat Rev Clin Oncol. 2020 Dec;17(12):725-741. doi: 10.1038/s41571-020-0413-z. Epub 2020 Aug 5. Nat Rev Clin Oncol. 2020. PMID: 32760014 Review.
-
Using the TCGA Database to Predict and Analyze Tumor Microenvironment Genes Related to Poor Prognosis of Colon Cancer.Med Sci Monit. 2020 Jun 18;26:e923707. doi: 10.12659/MSM.923707. Med Sci Monit. 2020. PMID: 32555128 Free PMC article.
-
Single-cell transcriptomics reveals the role of Macrophage-Naïve CD4 + T cell interaction in the immunosuppressive microenvironment of primary liver carcinoma.J Transl Med. 2022 Oct 11;20(1):466. doi: 10.1186/s12967-022-03675-2. J Transl Med. 2022. PMID: 36221095 Free PMC article.
-
Identification and validation of immunotherapy for four novel clusters of colorectal cancer based on the tumor microenvironment.Front Immunol. 2022 Oct 28;13:984480. doi: 10.3389/fimmu.2022.984480. eCollection 2022. Front Immunol. 2022. PMID: 36389763 Free PMC article.
-
Digital Immunophenotyping Predicts Disease Free and Overall Survival in Early Stage Melanoma Patients.Cells. 2021 Feb 17;10(2):422. doi: 10.3390/cells10020422. Cells. 2021. PMID: 33671367 Free PMC article.
References
-
- Aharinejad S, Salama M, Paulus P, Zins K, Berger A, Singer CF. Elevated CSF1 serum concentration predicts poor overall survival in women with early breast cancer. Endocrine-related cancer. 2013;20:777–783. - PubMed
-
- Apetoh L, Ghiringhelli F, Tesniere A, Criollo A, Ortiz C, Lidereau R, Mariette C, Chaput N, Mira JP, Delaloge S, et al. The interaction between HMGB1 and TLR4 dictates the outcome of anticancer chemotherapy and radiotherapy. Immunological reviews. 2007;220:47–59. - PubMed
-
- Appay V, Douek DC, Price DA. CD8+ T cell efficacy in vaccination and disease. Nat Med. 2008;14:623–628. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

