Plasmodium falciparum: multifaceted resistance to artemisinins
- PMID: 26955948
- PMCID: PMC4784301
- DOI: 10.1186/s12936-016-1206-9
Plasmodium falciparum: multifaceted resistance to artemisinins
Abstract
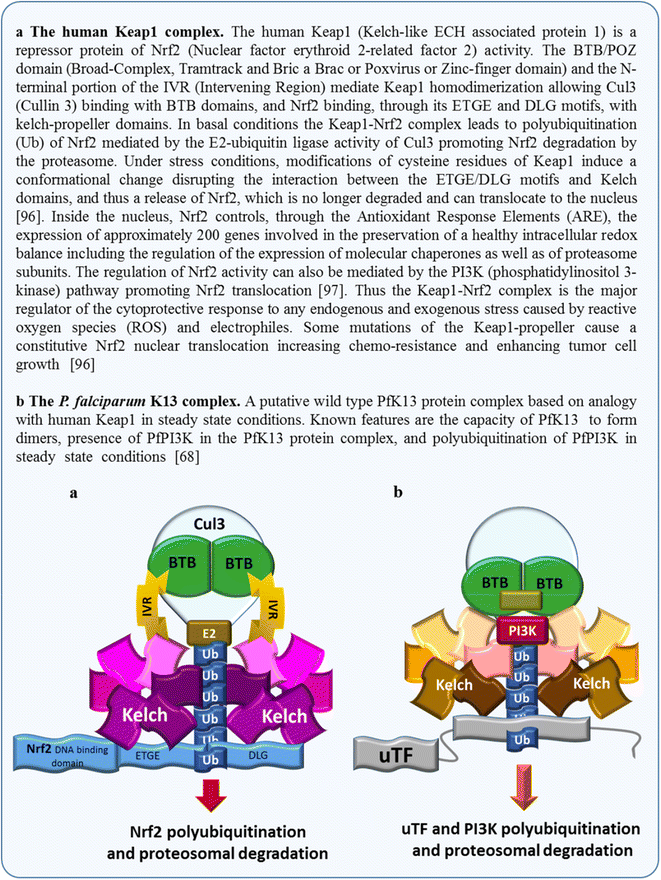
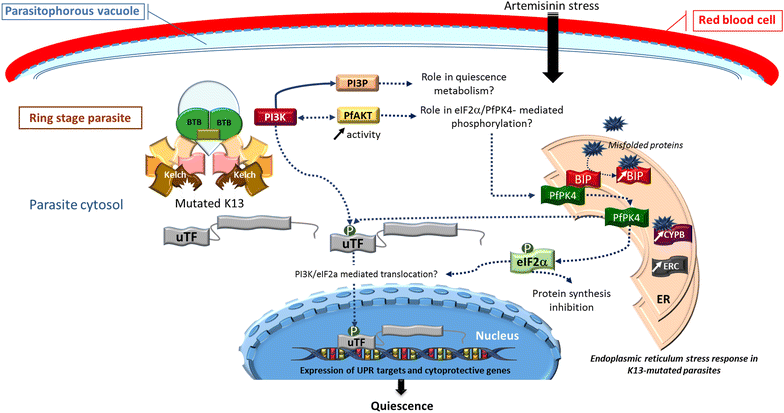
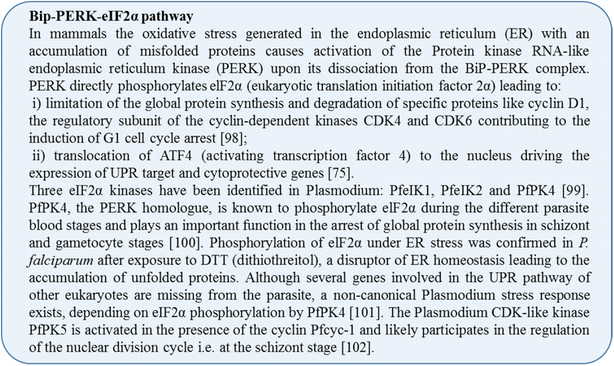
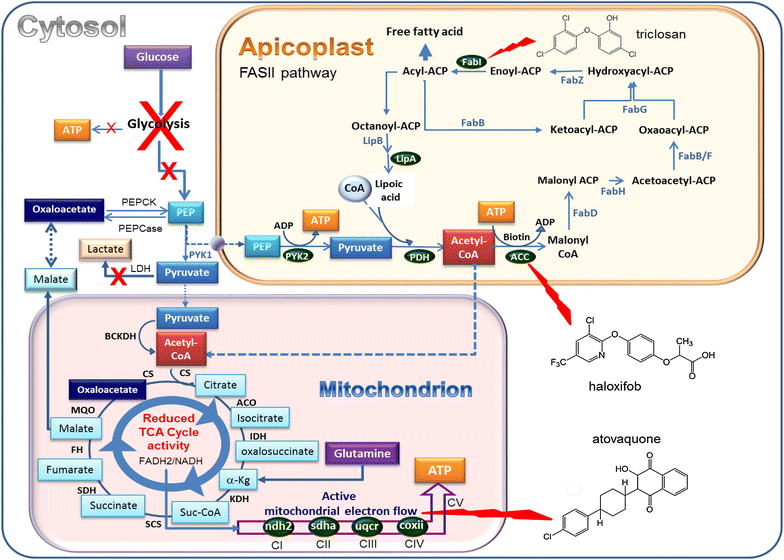
Plasmodium falciparum resistance to artemisinins, the most potent and fastest acting anti-malarials, threatens malaria elimination strategies. Artemisinin resistance is due to mutation of the PfK13 propeller domain and involves an unconventional mechanism based on a quiescence state leading to parasite recrudescence as soon as drug pressure is removed. The enhanced P. falciparum quiescence capacity of artemisinin-resistant parasites results from an increased ability to manage oxidative damage and an altered cell cycle gene regulation within a complex network involving the unfolded protein response, the PI3K/PI3P/AKT pathway, the PfPK4/eIF2α cascade and yet unidentified transcription factor(s), with minimal energetic requirements and fatty acid metabolism maintained in the mitochondrion and apicoplast. The detailed study of these mechanisms offers a way forward for identifying future intervention targets to fend off established artemisinin resistance.
Figures
Similar articles
-
Role of Plasmodium falciparum Kelch 13 Protein Mutations in P. falciparum Populations from Northeastern Myanmar in Mediating Artemisinin Resistance.mBio. 2020 Feb 25;11(1):e01134-19. doi: 10.1128/mBio.01134-19. mBio. 2020. PMID: 32098812 Free PMC article.
-
A molecular mechanism of artemisinin resistance in Plasmodium falciparum malaria.Nature. 2015 Apr 30;520(7549):683-7. doi: 10.1038/nature14412. Epub 2015 Apr 15. Nature. 2015. PMID: 25874676 Free PMC article.
-
Artemisinin Action and Resistance in Plasmodium falciparum.Trends Parasitol. 2016 Sep;32(9):682-696. doi: 10.1016/j.pt.2016.05.010. Epub 2016 Jun 9. Trends Parasitol. 2016. PMID: 27289273 Free PMC article. Review.
-
Drug resistance. Population transcriptomics of human malaria parasites reveals the mechanism of artemisinin resistance.Science. 2015 Jan 23;347(6220):431-5. doi: 10.1126/science.1260403. Epub 2014 Dec 11. Science. 2015. PMID: 25502316 Free PMC article.
-
A brief history of artemisinin: Modes of action and mechanisms of resistance.Chin J Nat Med. 2019 May 20;17(5):331-336. doi: 10.1016/S1875-5364(19)30038-X. Chin J Nat Med. 2019. PMID: 31171267 Review.
Cited by
-
Phosphatidylinositol 3-phosphate and Hsp70 protect Plasmodium falciparum from heat-induced cell death.Elife. 2020 Sep 25;9:e56773. doi: 10.7554/eLife.56773. Elife. 2020. PMID: 32975513 Free PMC article.
-
Targeting Artemisinin-Resistant Malaria by Repurposing the Anti-Hepatitis C Virus Drug Alisporivir.Antimicrob Agents Chemother. 2022 Dec 20;66(12):e0039222. doi: 10.1128/aac.00392-22. Epub 2022 Nov 14. Antimicrob Agents Chemother. 2022. PMID: 36374050 Free PMC article.
-
Oral Ingestion of Cannabis sativa: Risks, Benefits, and Effects on Malaria-Infected Hosts.Cannabis Cannabinoid Res. 2018 Nov 26;3(1):219-227. doi: 10.1089/can.2018.0043. eCollection 2018. Cannabis Cannabinoid Res. 2018. PMID: 30498786 Free PMC article.
-
Antimalarial Drug Predictions Using Molecular Descriptors and Machine Learning against Plasmodium Falciparum.Biomolecules. 2021 Nov 24;11(12):1750. doi: 10.3390/biom11121750. Biomolecules. 2021. PMID: 34944394 Free PMC article.
-
Rationalizing artemisinin-based combination therapies use for treatment of uncomplicated malaria: A situation analysis in health facilities and private pharmacies of Douala 5e-Cameroon.PLoS One. 2024 May 7;19(5):e0299517. doi: 10.1371/journal.pone.0299517. eCollection 2024. PLoS One. 2024. PMID: 38713730 Free PMC article.
References
-
- Nobel price. http://www.nobelprize.org/nobel_prizes/medicine/laureates/2015/. 2015.
-
- WHO . Status report on artemisinin and ACT resistance. Geneva: World Health Organization; 2015.
-
- Duru V, Khim N, Leang R, Kim S, Domergue A, Kloeung N, et al. Plasmodium falciparum dihydroartemisinin-piperaquine failures in Cambodia are associated with mutant K13 parasites presenting high survival rates in novel piperaquine in vitro assays: retrospective and prospective investigations. BMC Med. 2015;13:305. doi: 10.1186/s12916-015-0539-5. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

