Cytomegalovirus viral load and mortality after haemopoietic stem cell transplantation in the era of pre-emptive therapy: a retrospective cohort study
- PMID: 26947200
- PMCID: PMC4914379
- DOI: 10.1016/S2352-3026(15)00289-6
Cytomegalovirus viral load and mortality after haemopoietic stem cell transplantation in the era of pre-emptive therapy: a retrospective cohort study
Abstract
Background: Although cytomegalovirus viral load is commonly used to guide pre-emptive therapy in the post-transplantation setting, few data are available correlating viraemia with clinical endpoints. We therefore investigated the association between cytomegalovirus viral load and mortality in the first year after haemopoietic stem cell transplantation.
Methods: In this retrospective cohort study, we included patients from the Fred Hutchinson Cancer Research Center, WA, USA, who received an allogeneic haemopoietic stem cell transplantation between Jan 1, 2007, and Feb 28, 2013, were cytomegalovirus seropositive or had a seropositive donor, and underwent weekly plasma cytomegalovirus monitoring by PCR through to day 100 post-transplantation. Cox proportional hazards models were used to estimate the association of cytomegalovirus viral load at different thresholds with overall mortality by 1 year post-transplantation, adjusting for the use of pre-emptive therapy and other factors such as neutropenia, and graft-versus-host disease.
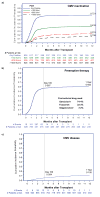
Findings: Of the 1037 patients initially selected for inclusion in this cohort, 87 (8%) patients were excluded because of missing cytomegalovirus testing and 24 (2%) were excluded because of their participation in cytomegalovirus prophylaxis trials. In the remaining 926 patients included in this study, the cumulative overall mortality was 30·0% (95% CI 26·9-33·0) 1 year after haemopoietic stem cell transplantation. 95 patients developed cytomegalovirus disease; death was directly attributable to cytomegalovirus disease in three (1%) of 263 patients who died in the first year after transplantation. A cytomegalovirus viral load of 250 IU/mL or greater was associated with increased risk of early (day 0-60 post-transplantation) death (adjusted hazard ratio [HR] 19·8, 95% CI 9·6-41·1). The risk was attenuated after day 60 (adjusted HR 1·8, 95% CI 1·3-2·3). Similar associations were noted for higher cytomegalovirus viral load thresholds.
Interpretation: Cytomegalovirus viraemia is associated with an increased risk of overall mortality in the first year after haemopoietic stem cell transplantation, independent of the use of pre-emptive therapy, and with evidence of a positive dose-response relationship. These data indicate the suitability of viral load as a surrogate clinical endpoint for clinical trials for cytomegalovirus vaccines, biologics, and drugs.
Funding: Merck and Co, National Institutes of Health.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Dr. Green reports grants from Merck & Co., Inc. during the conduct of the study; grants and personal fees from Astellas outside the submitted work. Dr. Leisenring and Ms. Xie received grants from Merck & Co., Inc. for the conduct of the study. Drs. Mast, Cui, and Marks are current employees of, and own stock in Merck and Co. Inc. Dr. Sorror reports personal fees from Jazz Pharmaceuticals, outside the submitted work. Dr. Boeckh reports grants and personal fees from Merck & Co., Inc., during the conduct of the study; grants and personal fees from Astellas, Shire, Roche/Genentech, Gilead and Chimerix; personal fees from Clinigen and Microbiotix, outside the submitted work. Drs. Sandmaier, Goyal, Özkök, Sahoo, Kimball, Jerome, and Ms. Yi have nothing to disclose.
Figures
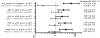


Comment in
-
Does cytomegalovirus viral load in stem-cell transplant recipients matter?Lancet Haematol. 2016 Mar;3(3):e102. doi: 10.1016/S2352-3026(16)00022-3. Epub 2016 Feb 20. Lancet Haematol. 2016. PMID: 26947196 No abstract available.
Similar articles
-
Addition of sirolimus to standard cyclosporine plus mycophenolate mofetil-based graft-versus-host disease prophylaxis for patients after unrelated non-myeloablative haemopoietic stem cell transplantation: a multicentre, randomised, phase 3 trial.Lancet Haematol. 2019 Aug;6(8):e409-e418. doi: 10.1016/S2352-3026(19)30088-2. Epub 2019 Jun 24. Lancet Haematol. 2019. PMID: 31248843 Free PMC article. Clinical Trial.
-
A novel therapeutic cytomegalovirus DNA vaccine in allogeneic haemopoietic stem-cell transplantation: a randomised, double-blind, placebo-controlled, phase 2 trial.Lancet Infect Dis. 2012 Apr;12(4):290-9. doi: 10.1016/S1473-3099(11)70344-9. Epub 2012 Jan 10. Lancet Infect Dis. 2012. PMID: 22237175 Clinical Trial.
-
Cytomegalovirus pre-emptive therapy after hematopoietic stem cell transplantation in the era of real-time quantitative PCR: comparison with recipients of solid organ transplants.Transpl Infect Dis. 2016 Jun;18(3):405-14. doi: 10.1111/tid.12542. Epub 2016 Jun 9. Transpl Infect Dis. 2016. PMID: 27061703
-
Oral valganciclovir versus ganciclovir as delayed pre-emptive therapy for patients after allogeneic hematopoietic stem cell transplant: a pilot trial (04-0274) and review of the literature.Transpl Infect Dis. 2012 Jun;14(3):259-67. doi: 10.1111/j.1399-3062.2011.00689.x. Epub 2011 Oct 28. Transpl Infect Dis. 2012. PMID: 22093134 Review.
-
CMV-specific immune reconstitution following allogeneic stem cell transplantation.Virulence. 2016 Nov 16;7(8):967-980. doi: 10.1080/21505594.2016.1221022. Epub 2016 Aug 9. Virulence. 2016. PMID: 27580355 Free PMC article. Review.
Cited by
-
Refractory and Resistant Cytomegalovirus After Hematopoietic Cell Transplant in the Letermovir Primary Prophylaxis Era.Clin Infect Dis. 2021 Oct 20;73(8):1346-1354. doi: 10.1093/cid/ciab298. Clin Infect Dis. 2021. PMID: 33830182 Free PMC article.
-
Outcomes of allogeneic hematopoietic cell transplantation under letermovir prophylaxis for cytomegalovirus infection.Ann Hematol. 2024 Jan;103(1):285-296. doi: 10.1007/s00277-023-05474-1. Epub 2023 Nov 10. Ann Hematol. 2024. PMID: 37947825
-
Letermovir and its role in the prevention of cytomegalovirus infection in seropositive patients receiving an allogeneic hematopoietic cell transplant.Ther Adv Hematol. 2020 Jun 24;11:2040620720937150. doi: 10.1177/2040620720937150. eCollection 2020. Ther Adv Hematol. 2020. PMID: 32637057 Free PMC article. Review.
-
Low-Level Cytomegalovirus Antigenemia Promotes Protective Cytomegalovirus Antigen-Specific T Cells after Allogeneic Hematopoietic Cell Transplantation.Biol Blood Marrow Transplant. 2020 Nov;26(11):2147-2154. doi: 10.1016/j.bbmt.2020.07.024. Epub 2020 Jul 25. Biol Blood Marrow Transplant. 2020. PMID: 32721522 Free PMC article.
-
The promising efficacy of a risk-based letermovir use strategy in CMV-positive allogeneic hematopoietic cell recipients.Blood Adv. 2023 Mar 14;7(5):856-865. doi: 10.1182/bloodadvances.2022008667. Blood Adv. 2023. PMID: 36350752 Free PMC article.
References
-
- Marty FM, Ljungman P, Papanicolaou GA, et al. Maribavir prophylaxis for prevention of cytomegalovirus disease in recipients of allogeneic stem-cell transplants: a phase 3, double-blind, placebo-controlled, randomised trial. The Lancet Infectious Diseases. 2011;11:284–92. - PubMed
-
- Marty FM, Winston DJ, Rowley SD, et al. CMX001 to Prevent Cytomegalovirus Disease in Hematopoietic-Cell Transplantation. New England Journal of Medicine. 2013;369:1227–36. - PubMed
-
- Chemaly RF, Ullmann AJ, Stoelben S, et al. Letermovir for Cytomegalovirus Prophylaxis in Hematopoietic-Cell Transplantation. New England Journal of Medicine. 2014;370:1781–9. - PubMed
-
- Ljungman P, Perez-Bercoff L, Jonsson J, et al. Risk factors for the development of cytomegalovirus disease after allogeneic stem cell transplantation. Haematologica. 2006;91:78–83. - PubMed
-
- Gerna G, Lilleri D, Caldera D, Furione M, Zenone Bragotti L, Alessandrino EP. Validation of a DNAemia cutoff for preemptive therapy of cytomegalovirus infection in adult hematopoietic stem cell transplant recipients. Bone Marrow Transplant. 2008;41:873–9. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

