Overexpression of pig selenoprotein S blocks OTA-induced promotion of PCV2 replication by inhibiting oxidative stress and p38 phosphorylation in PK15 cells
- PMID: 26943035
- PMCID: PMC4991468
- DOI: 10.18632/oncotarget.7814
Overexpression of pig selenoprotein S blocks OTA-induced promotion of PCV2 replication by inhibiting oxidative stress and p38 phosphorylation in PK15 cells
Abstract
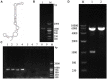
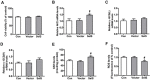
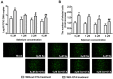
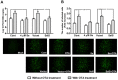
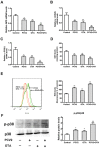
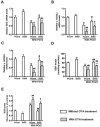
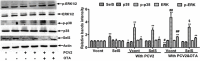
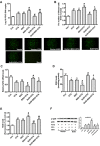
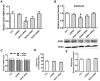
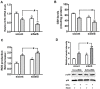
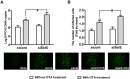
Porcine circovirus type 2 (PCV2) is the primary cause of porcine circovirus disease, and ochratoxin A (OTA)-induced oxidative stress promotes PCV2 replication. In humans, selenoprotein S (SelS) has antioxidant ability, but it is unclear whether SelS affects viral infection. Here, we stably transfected PK15 cells with pig pCDNA3.1-SelS to overexpress SelS. Selenium (Se) at 2 or 4 μM and SelS overexpression blocked the OTA-induced increases of PCV2 DNA copy number and infected cell numbers. SelS overexpression also increased glutathione (GSH), NF-E2-related factor 2 (Nrf2) mRNA, and γ-glutamyl-cysteine synthetase mRNA levels; decreased reactive oxygen species (ROS) levels; and inhibited p38 phosphorylation in PCV2-infected PK15 cells, regardless of OTA treatment. Buthionine sulfoximine reversed all of the above SelS-induced changes. siRNA-mediated SelS knockdown decreased Nrf2 mRNA and GSH levels, increased ROS levels, and promoted PCV2 replication in OTA-treated PK15 cells. These data indicate that pig SelS blocks OTA-induced promotion of PCV2 replication by inhibiting the oxidative stress and p38 phosphorylation in PK15 cells.
Keywords: ochratoxin A; overexpression of selenoprotein S; oxidative stress; p38 signaling pathway; porcine circovirus type 2.
Conflict of interest statement
All authors declare no financial conflict of interest.
Figures

Similar articles
-
Overexpression and Low Expression of Selenoprotein S Impact Ochratoxin A-Induced Porcine Cytotoxicity and Apoptosis in Vitro.J Agric Food Chem. 2017 Aug 16;65(32):6972-6981. doi: 10.1021/acs.jafc.7b02115. Epub 2017 Aug 7. J Agric Food Chem. 2017. PMID: 28650663
-
Ochratoxin A promotes porcine circovirus type 2 replication in vitro and in vivo.Free Radic Biol Med. 2015 Mar;80:33-47. doi: 10.1016/j.freeradbiomed.2014.12.016. Epub 2014 Dec 24. Free Radic Biol Med. 2015. PMID: 25542137 Free PMC article.
-
Reactive oxygen species regulate the replication of porcine circovirus type 2 via NF-κB pathway.Virology. 2012 Apr 25;426(1):66-72. doi: 10.1016/j.virol.2012.01.023. Epub 2012 Feb 11. Virology. 2012. PMID: 22330204
-
Interactions of porcine circovirus 2 with its hosts.Virus Genes. 2016 Aug;52(4):437-44. doi: 10.1007/s11262-016-1326-x. Epub 2016 Mar 25. Virus Genes. 2016. PMID: 27016220 Review.
-
Porcine circovirus type 2 (PCV2): pathogenesis and interaction with the immune system.Annu Rev Anim Biosci. 2013 Jan;1:43-64. doi: 10.1146/annurev-animal-031412-103720. Epub 2013 Jan 3. Annu Rev Anim Biosci. 2013. PMID: 25387012 Review.
Cited by
-
Regulation of Apoptosis During Porcine Circovirus Type 2 Infection.Front Microbiol. 2018 Sep 4;9:2086. doi: 10.3389/fmicb.2018.02086. eCollection 2018. Front Microbiol. 2018. PMID: 30233552 Free PMC article. Review.
-
Selenoprotein S: a therapeutic target for diabetes and macroangiopathy?Cardiovasc Diabetol. 2017 Aug 10;16(1):101. doi: 10.1186/s12933-017-0585-8. Cardiovasc Diabetol. 2017. PMID: 28797256 Free PMC article. Review.
-
Selenium, Selenoproteins and Viral Infection.Nutrients. 2019 Sep 4;11(9):2101. doi: 10.3390/nu11092101. Nutrients. 2019. PMID: 31487871 Free PMC article. Review.
-
Pattern-Recognition Receptor Agonist-Containing Immunopotentiator CVC1302 Boosts High-Affinity Long-Lasting Humoral Immunity.Front Immunol. 2021 Nov 18;12:697292. doi: 10.3389/fimmu.2021.697292. eCollection 2021. Front Immunol. 2021. PMID: 34867941 Free PMC article.
-
Risk assessment of ochratoxin A in food.EFSA J. 2020 May 13;18(5):e06113. doi: 10.2903/j.efsa.2020.6113. eCollection 2020 May. EFSA J. 2020. PMID: 37649524 Free PMC article.
References
-
- Segales J. Porcine circovirus type 2 (PCV2) infections: Clinical signs, pathology and laboratory diagnosis. Virus Res. 2012;164:10–19. - PubMed
-
- Grau-Roma L, Fraile L, Segales J. Recent advances in the epidemiology, diagnosis and control of diseases caused by porcine circovirus type 2. Vet J. 2011;187:23–32. - PubMed
-
- Chen X, Ren F, Hesketh J, Shi X, Li J, Gan F, Huang K. Reactive oxygen species regulate the replication of porcine circovirus type 2 via NF-kappa B pathway. Virology. 2012;426:66–72. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

